Anti- Helicobacter pylori, anti-biofilm activity, and molecular docking study of citropten, bergapten, and its positional isomer isolated from Citrus sinensis L. leaves
- PMID: 38352786
- PMCID: PMC10861955
- DOI: 10.1016/j.heliyon.2024.e25232
Anti- Helicobacter pylori, anti-biofilm activity, and molecular docking study of citropten, bergapten, and its positional isomer isolated from Citrus sinensis L. leaves
Abstract
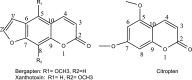
Introduction: Citrus sinensis L. is a candidate plant with promising antimicrobial potential. In the current study, the phytochemical investigation of C. sinensis leaf extract led to the isolation of three coumarins, namely bergapten, xanthotoxin, and citropten.
Methods: The chemical structures of the isolated coumarins were elucidated using NMR and ESI-MS techniques. The total aqueous ethanol leaf extract and the isolated coumarins were evaluated for their antimicrobial effects against Helicobacter pylori using the MTT-micro-well dilution method and its anti-biofilm activity using MBEC assay, as compared to clarithromycin.
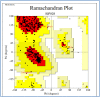
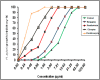
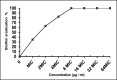
Results: The results showed that citropten scored the lowest MIC value at 3.9 μg/mL and completely inhibited the planktonic growth of H. pylori. In addition, it completely suppressed H. pylori biofilm at 31.25 μg/mL. These findings have been supported by molecular docking studies on the active sites of the H. pylori inosine 5'-monophosphate dehydrogenase (HpIMPDH) model and the urease enzyme, showing a strong binding affinity of citropten to HpIMPDH with seven hydrogen bonds and a binding energy of -6.9 kcal/mol. Xanthotoxin and bergapten showed good docking scores, both at -6.5 kcal/mol for HpIMPDH, with each having four hydrogen bondings. Furthermore, xanthotoxin showed many hydrophobic interactions, while bergapten formed one Pi-anion interaction. Concerning docking in the urease enzyme, the compounds showed mild to moderate binding affinities as compared to the ligand. Thus, based on docking results and good binding scores observed with the HpIMPDH active site, an in-vitro HpIMPDH inhibition assay was done for the compounds. Citropten showed the most promising inhibitory activity with an IC50 value of 2.4 μM. Conclusion: The present study demonstrates that C. sinensis L. leaves are a good source for supplying coumarins that can act as naturally effective anti-H. pylori agents.
Keywords: Bergapten; Citropten; Citrus sinensis; Helicobacter pylori; HpIMPDH; Molecular docking; Urease; Xanthotoxin.
© 2024 The Authors. Published by Elsevier Ltd.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



Similar articles
-
Irigenin, a novel lead from Iris confusa for management of Helicobacter pylori infection with selective COX-2 and HpIMPDH inhibitory potential.Sci Rep. 2022 Jul 6;12(1):11457. doi: 10.1038/s41598-022-15361-w. Sci Rep. 2022. PMID: 35794127 Free PMC article.
-
Design, synthesis, and biological evaluation of Helicobacter pylori inosine 5'-monophosphate dehydrogenase (HpIMPDH) inhibitors.Drug Dev Res. 2019 Feb;80(1):125-132. doi: 10.1002/ddr.21467. Epub 2018 Nov 1. Drug Dev Res. 2019. PMID: 30381846
-
Development, synthesis, and biological evaluation of sulfonyl-α-l-amino acids as potential anti-Helicobacter pylori and IMPDH inhibitors.Arch Pharm (Weinheim). 2021 Jun;354(6):e2000385. doi: 10.1002/ardp.202000385. Epub 2021 Feb 12. Arch Pharm (Weinheim). 2021. PMID: 33576040
-
Design, synthesis and biological evaluation of Helicobacter pylori inosine 5'-monophosphate dehydrogenase (HpIMPDH) inhibitors. Further optimization of selectivity towards HpIMPDH over human IMPDH2.Bioorg Chem. 2019 Jun;87:753-764. doi: 10.1016/j.bioorg.2019.04.001. Epub 2019 Apr 4. Bioorg Chem. 2019. PMID: 30974298
-
Methoxyfuranocoumarins of Natural Origin-Updating Biological Activity Research and Searching for New Directions-A Review.Curr Issues Mol Biol. 2024 Jan 19;46(1):856-883. doi: 10.3390/cimb46010055. Curr Issues Mol Biol. 2024. PMID: 38275669 Free PMC article. Review.
Cited by
-
Anti-urease therapy: a targeted approach to mitigating antibiotic resistance in Helicobacter pylori while preserving the gut microflora.Gut Pathog. 2025 May 28;17(1):37. doi: 10.1186/s13099-025-00708-1. Gut Pathog. 2025. PMID: 40437630 Free PMC article. Review.
-
Insights into Novel Arylazopyrazolo[1,5-a]pyrimidines as Promising MurA Inhibitors and Antibiofilm Candidates: Design, Synthesis, Antimicrobial Evaluation, and Molecular Docking.ACS Omega. 2025 Jan 24;10(4):4044-4056. doi: 10.1021/acsomega.4c10286. eCollection 2025 Feb 4. ACS Omega. 2025. PMID: 39926530 Free PMC article.
-
Etrog Citron (Citrus medica) as a Novel Source of Antimicrobial Agents: Overview of Its Bioactive Phytochemicals and Delivery Approaches.Pharmaceutics. 2025 Jun 9;17(6):761. doi: 10.3390/pharmaceutics17060761. Pharmaceutics. 2025. PMID: 40574073 Free PMC article. Review.
-
Challenges and Prospects for Eradication of Helicobacter pylori: Targeting Virulence Factors, Metabolism, and Vaccine Innovation.Pathogens. 2025 Jun 21;14(7):619. doi: 10.3390/pathogens14070619. Pathogens. 2025. PMID: 40732667 Free PMC article. Review.
-
A review of the ethnomedicinal, phytochemical, and pharmacological properties of the Ferulago genus based on Structure-Activity Relationship (SAR) of coumarins.Daru. 2024 Dec;32(2):825-899. doi: 10.1007/s40199-024-00530-1. Epub 2024 Aug 19. Daru. 2024. PMID: 39158662 Free PMC article. Review.
References
-
- McConaghy J.R., Decker A., Nair S. Peptic ulcer disease and H. pylori infection: common questions and answers. Am. Fam. Physician. 2023;107:165–172. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

