Intravenous immunoglobulin for chronic inflammatory demyelinating polyradiculoneuropathy
- PMID: 38353301
- PMCID: PMC10865446
- DOI: 10.1002/14651858.CD001797.pub4
Intravenous immunoglobulin for chronic inflammatory demyelinating polyradiculoneuropathy
Abstract
Background: Chronic inflammatory demyelinating polyradiculoneuropathy (CIDP) causes progressive or relapsing weakness and numbness of the limbs, which lasts for at least two months. Uncontrolled studies have suggested that intravenous immunoglobulin (IVIg) could help to reduce symptoms. This is an update of a review first published in 2002 and last updated in 2013.
Objectives: To assess the efficacy and safety of intravenous immunoglobulin in people with chronic inflammatory demyelinating polyradiculoneuropathy.
Search methods: We searched the Cochrane Neuromuscular Specialised Register, CENTRAL, MEDLINE, Embase, and two trials registers on 8 March 2023.
Selection criteria: We selected randomised controlled trials (RCTs) and quasi-RCTs that tested any dose of IVIg versus placebo, plasma exchange, or corticosteroids in people with definite or probable CIDP.
Data collection and analysis: We used standard Cochrane methods. Our primary outcome was significant improvement in disability within six weeks after the start of treatment, as determined and defined by the study authors. Our secondary outcomes were change in mean disability score within six weeks, change in muscle strength (Medical Research Council (MRC) sum score) within six weeks, change in mean disability score at 24 weeks or later, frequency of serious adverse events, and frequency of any adverse events. We used GRADE to assess the certainty of evidence for our main outcomes.
Main results: We included nine RCTs with 372 participants (235 male) from Europe, North America, South America, and Israel. There was low statistical heterogeneity between the trial results, and the overall risk of bias was low for all trials that contributed data to the analysis. Five trials (235 participants) compared IVIg with placebo, one trial (20 participants) compared IVIg with plasma exchange, two trials (72 participants) compared IVIg with prednisolone, and one trial (45 participants) compared IVIg with intravenous methylprednisolone (IVMP). We included one new trial in this update, though it contributed no data to any meta-analyses. IVIg compared with placebo increases the probability of significant improvement in disability within six weeks of the start of treatment (risk ratio (RR) 2.40, 95% confidence interval (CI) 1.72 to 3.36; number needed to treat for an additional beneficial outcome (NNTB) 4, 95% CI 3 to 5; 5 trials, 269 participants; high-certainty evidence). Since each trial used a different disability scale and definition of significant improvement, we were unable to evaluate the clinical relevance of the pooled effect. IVIg compared with placebo improves disability measured on the Rankin scale (0 to 6, lower is better) two to six weeks after the start of treatment (mean difference (MD) -0.26 points, 95% CI -0.48 to -0.05; 3 trials, 90 participants; high-certainty evidence). IVIg compared with placebo probably improves disability measured on the Inflammatory Neuropathy Cause and Treatment (INCAT) scale (1 to 10, lower is better) after 24 weeks (MD 0.80 points, 95% CI 0.23 to 1.37; 1 trial, 117 participants; moderate-certainty evidence). There is probably little or no difference between IVIg and placebo in the frequency of serious adverse events (RR 0.82, 95% CI 0.36 to 1.87; 3 trials, 315 participants; moderate-certainty evidence). The trial comparing IVIg with plasma exchange reported none of our main outcomes. IVIg compared with prednisolone probably has little or no effect on the probability of significant improvement in disability four weeks after the start of treatment (RR 0.91, 95% CI 0.50 to 1.68; 1 trial, 29 participants; moderate-certainty evidence), and little or no effect on change in mean disability measured on the Rankin scale (MD 0.21 points, 95% CI -0.19 to 0.61; 1 trial, 24 participants; moderate-certainty evidence). There is probably little or no difference between IVIg and prednisolone in the frequency of serious adverse events (RR 0.45, 95% CI 0.04 to 4.69; 1 cross-over trial, 32 participants; moderate-certainty evidence). IVIg compared with IVMP probably increases the likelihood of significant improvement in disability two weeks after starting treatment (RR 1.46, 95% CI 0.40 to 5.38; 1 trial, 45 participants; moderate-certainty evidence). IVIg compared with IVMP probably has little or no effect on change in disability measured on the Rankin scale two weeks after the start of treatment (MD 0.24 points, 95% CI -0.15 to 0.63; 1 trial, 45 participants; moderate-certainty evidence) or on change in mean disability measured with the Overall Neuropathy Limitation Scale (ONLS, 1 to 12, lower is better) 24 weeks after the start of treatment (MD 0.03 points, 95% CI -0.91 to 0.97; 1 trial, 45 participants; moderate-certainty evidence). The frequency of serious adverse events may be higher with IVIg compared with IVMP (RR 4.40, 95% CI 0.22 to 86.78; 1 trial, 45 participants, moderate-certainty evidence).
Authors' conclusions: Evidence from RCTs shows that IVIg improves disability for at least two to six weeks compared with placebo, with an NNTB of 4. During this period, IVIg probably has similar efficacy to oral prednisolone and IVMP. Further placebo-controlled trials are unlikely to change these conclusions. In one large trial, the benefit of IVIg compared with placebo in terms of improved disability score persisted for 24 weeks. Further research is needed to assess the long-term benefits and harms of IVIg relative to other treatments.
Trial registration: ClinicalTrials.gov NCT01349270 NCT02638207 NCT01225276 NCT03684018.
Copyright © 2024 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
SB: reports no competing interests. RdH: reports no competing interests. MV: reports no competing interests. INvS: chairs a steering committee for CSL‐Behring and received departmental honoraria for serving on scientific advisory boards for CSL‐Behring and UCB. He received speakers' fees from CSL‐Behring. Departmental research support has been granted by The Netherlands Organisation for Scientific Research, and the Dutch Prinses Beatrix Spierfonds. All lecturing and consulting fees for INvS were donated to the Stichting Klinische Neurologie, a local foundation that supports research in the field of neurological disorders. INvS is a member of the Scientific Board of the Kreuth III meeting on the optimal use of plasma‐derived medicinal products, especially coagulation factors and normal immunoglobulins organised under the auspices of the European Directorate for the Quality of Medicines & HealthCare (EDQM). FE: received support for printing of his thesis from Sanquin Bloedvoorziening (Dutch blood bank and IVIg manufacturer) and CSL‐Behring in 2014. He reports lecture fees from CSL‐Behring, Kedrion, and Grifols. Outside the submitted work, as principal investigator of INCbase, FE also reports investigator‐initiated grants from Kedrion, Terumo BCT, CSL‐Behring, and Takeda Pharmaceutical Company, and grants from ZonMw (Dutch governmental agency) and Prinses Beatrix Spierfonds (a Dutch charity). In addition, his institution has received fees from UCB Pharma, CSL‐Behring, and Takeda for advisory board membership. All grants and fees were paid to his institution. He is a member of the Cochrane Neuromuscular Editorial Board.
Figures
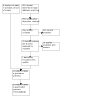
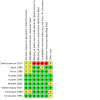

























Update of
-
Intravenous immunoglobulin for chronic inflammatory demyelinating polyradiculoneuropathy.Cochrane Database Syst Rev. 2013 Dec 30;(12):CD001797. doi: 10.1002/14651858.CD001797.pub3. Cochrane Database Syst Rev. 2013. Update in: Cochrane Database Syst Rev. 2024 Feb 14;2:CD001797. doi: 10.1002/14651858.CD001797.pub4. PMID: 24379104 Updated.
References
References to studies included in this review
Camdessanche 2014 {published and unpublished data}
-
- Camdessanche JP, Ferraud K, Lagrange E, Viala K, Chan V, Guy Renouil N, et al. Multicentre, randomised, open-label trial to compare efficacy and tolerance of prednisone and IVIG In patients with CIDP on a one year follow up (P7.092). Neurology 2014;82(10 Suppl):P7.092.
Dyck 1994 {published data only}
-
- Dyck PJ, Litchy WJ, Kratz KM, Suarez GA, Low PA, Pineda AA, et al. A plasma exchange versus immune globulin infusion trial in chronic inflammatory demyelinating polyradiculoneuropathy. Annals of Neurology 1994;36(6):838-45. [PMID: ] - PubMed
Hahn 1996 {published data only}
-
- Hahn AF, Bolton CF, Zochodne D, Feasby TE. Intravenous immunoglobulin treatment in chronic inflammatory demyelinating polyneuropathy. A double-blind, placebo-controlled, cross-over study. Brain 1996;119(Part 4):1067-77. [PMID: ] - PubMed
Hughes 2001 {published and unpublished data}
-
- Hughes R, Bensa S, Willison HJ, Van den Bergh P, Comi G, Illa I, et al. Randomized controlled trial of intravenous immunoglobulin versus oral prednisolone in chronic inflammatory demyelinating polyradiculoneuropathy. Annals of Neurology 2001;50(2):195-201. [PMID: ] - PubMed
Hughes 2008 {published and unpublished data}
-
- Hughes RA, Donofrio P, Bril V, Dalakas MC, Deng C, Hanna K, et al, ICE Study Group. Intravenous immune globulin (10% caprylate-chromatography purified) for the treatment of chronic inflammatory demyelinating polyradiculoneuropathy (ICE Study): a randomised placebo-controlled trial. Lancet Neurology 2008;7(2):136-44. [PMID: ] - PubMed
Mendell 2001 {published data only}
-
- Mendell JR, Barohn RJ, Freimer ML, Kissel JT, King WM, Nagaraja HN, et al. Randomized controlled trial of IVIg in untreated chronic inflammatory demyelinating polyradiculoneuropathy. Neurology 2001;56(4):445-9. [PMID: ] - PubMed
Nobile‐Orazio 2012 {published and unpublished data}
-
- Nobile-Orazio N, Cocito D, Jann S, Uncini A, Beghi E, Messina P, et al, IMC Trial Group. Intravenous immunoglobulin versus intravenous methylprednisolone for chronic inflammatory demyelinating polyradiculoneuropathy: a randomised controlled trial. Lancet Neurology 2012;11(6):493-502. [PMID: ] - PubMed
Thompson 1996 {published and unpublished data}
-
- Thompson N, Choudhary P, Hughes RA, Quinlivan RM. A novel trial design to study the effect of intravenous immunoglobulin in chronic inflammatory demyelinating polyradiculoneuropathy. Journal of Neurology 1996;243(3):280-5. [PMID: ] - PubMed
Vermeulen 1993 {published and unpublished data}
-
- Vermeulen M, Doorn PA, Brand A, Strengers PF, Jennekens FG, Busch HF. Intravenous immunoglobulin treatment in patients with chronic inflammatory demyelinating polyneuropathy: a double blind, placebo controlled study. Journal of Neurology, Neurosurgery & Psychiatry 1993;56(1):36-9. [PMID: ] - PMC - PubMed
References to studies excluded from this review
Baba 1996 {published data only}
-
- Baba M, Ogawa M, Matsunaga M. Treatment of chronic inflammatory demyelinating polyneuropathy (CIDP). Clinical Neurology 1996;36(12):1336-7. - PubMed
Breiner 2019 {published data only}
-
- Breiner A, Tapia CB, Lovblom LE, Perkins B, Katzberg H, Bril V. Intravenous immunoglobulin for treatment of demyelinating polyneuropathy in patients with diabetes mellitus: a randomized, double-blinded, placebo-controlled crossover trial. Neurology Neuroimmunology Neuroinflammation 2019;6(5):586. [DOI: 10.1212/NXI.0000000000000586] - DOI
Cornblath 2022 {published data only}
-
- NCT02638207. Study to evaluate safety and efficacy of three different dosages of NewGam in patients with chronic inflammatory demyelinating poly (radiculo) neuropathy (CIDP). clinicaltrials.gov/study/NCT02638207 (first received 23 December 2015).
Curro 1987 {published data only}
-
- Curro DB, Tezzon F. High-dose intravenous gammaglobulin for chronic inflammatory demyelinating polyneuropathy. Italian Journal of Neurological Sciences 1987;8(4):321-6. - PubMed
Dalakas 1996 {published data only}
-
- Dalakas MC, Quarles RH, Farrer RG, Dambrosia J, Soueidan S, Stein DP, et al. A controlled study of intravenous immunoglobulin in demyelinating neuropathy with IgM gammopathy. Annals of Neurology 1996;40(5):792-5. - PubMed
EudraCT2012‐001996‐34 {unpublished data only}https://www.clinicaltrialsregister.eu/ctr-search/search?query=2012-001996-34
-
- EudraCT2012-001996-34. A comparative, double-blind, randomised, multicentre efficacy and safety study of ClairYg® versus Tégéline® in maintenance treatment of chronic inflammatory demyelinating polyradiculoneuropathy (CIDP). clinicaltrialsregister.eu/ctr-search/trial/2012-001996-34/FR (first received 3 September 2012).
Hankey 1994 {published data only}
ISRCTN13637698 {unpublished data only}ISRCTN13637698
-
- ISRCTN13637698. Intravenous immunoglobulin overtreatment in chronic inflammatory demyelinating polyneuropathy. isrctn.com/ISRCTN13637698 (first received 6 December 2013). [DOI: ]
Kubori 1999 {published data only}
-
- Kubori T, Mezaki T, Kaji R, Kimura J, Hamaguchi K, Hirayama K, et al. The clinical usefulness of high-dose intravenous immunoglobulin therapy for chronic inflammatory demyelinating polyneuropathy and multifocal motor neuropathy. Brain & Nerve 1999;51(2):127-35. - PubMed
Kuitwaard 2021 {published data only}
-
- Kuitwaard K, Brusse E, Jacobs BC, Vrancken AFJE, Eftimov F, Notermans N, et al. Randomized trial of intravenous immunoglobulin maintenance treatment regimens in chronic inflammatory demyelinating polyradiculoneuropathy. European Journal of Neurology 2021;28(1):286-96. [DOI: 10.1111/ene.14501] - DOI - PMC - PubMed
Kuwabara 2017 {published data only}
-
- Kuwabara S, Mori M, Misawa S, Suzuki M, Nishiyama K, Mutoh T, et al. Intravenous immunoglobulin for maintenance treatment of chronic inflammatory demyelinating polyneuropathy: a multicentre, open-label, 52-week phase III trial. Journal of Neurology, Neurosurgery and Psychiatry 2017;88(10):832-8. [DOI: 10.1136/jnnp-2017-316427] - DOI - PMC - PubMed
Léger 2013 {published data only}
-
- Léger JM, De Bleecker JL, Sommer C, Robberecht W, Saarela M, Kamienowski J, et al. Efficacy and safety of Privigen in patients with chronic inflammatory demyelinating polyneuropathy: results of a prospective, single-arm, open-label Phase III study (the PRIMA study). Journal of the Peripheral Nervous System 2013;18(2):130-40. [DOI: 10.1111/jns5.12017] - DOI - PMC - PubMed
Markvardsen 2017 {published data only}
-
- Markvardsen LH, Sindrup SH, Christiansen I, Olsen NK, Jakobsen J, Andersen H, et al. Subcutaneous immunoglobulin as first-line therapy in treatment-naive patients with chronic inflammatory demyelinating polyneuropathy: randomized controlled trial study. European Journal of Neurology 2017;24(2):412-8. [DOI: 10.1111/ene.13218] - DOI - PubMed
NCT01225276 {published data only}
-
- NCT01225276. Safety and efficacy study of three different dosages of NewGam in patients with CIDP (POINT). clinicaltrials.gov/show/NCT01225276 (first received 20 October 2010).
NCT03684018 {unpublished data only}
-
- NCT03684018. Single vs. multiple Privigen dose regimens in pediatric CIDP. clinicaltrials.gov/ct2/show/NCT03684018 (first received 25 September 2018).
Neacsu 2016 {unpublished data only}
-
- Neacsu MG, Loan B, Buraga M, Baetu C, Cioriceanu IH, Ghitoiu AM, et al. CIDP - intravenous immunoglobulin or corticosteroids? European Journal of Neurology 2016;23(Suppl 2):737, Abstract no P31266.
van Doorn 1990b {published data only}
-
- Doorn PA, Brand A, Strengers PF, Meulstee J, Vermeulen M. High-dose intravenous immunoglobulin treatment in chronic inflammatory demyelinating polyneuropathy: a double-blind, placebo-controlled, crossover study. Neurology 1990;40(2):209-12. - PubMed
Zinman 2005 {published data only}
-
- Zinman LH, Sutton D, Ng E, Nwe P, Ngo M, Bril V. A pilot study to compare the use of Excorim staphylococcal protein immunoadsorption system and IVIG in chronic inflammatory demyelinating polyneuropathy. Transfusion and Apheresis Science 2005;33(3):317-24. - PubMed
Additional references
Abrams 2005
-
- Abrams KR, Gillies CL, Lambert PC. Meta-analysis of heterogeneously reported trials assessing change from baseline. Statistics in Medicine 2005;24(24):3823-44. - PubMed
Ad hoc subcom 1991
-
- Ad hoc subcommittee of the American Academy of Neurology AIDS Task Force. Research criteria for diagnosis of chronic inflammatory demyelinating polyneuropathy (CIDP). Neurology 1991;41(5):617-8. - PubMed
Barohn 1989
-
- Barohn RJ, Kissel JT, Warmolts JR, Mendell JR. Chronic inflammatory demyelinating polyradiculoneuropathy. Clinical characteristics, course, and recommendations for diagnostic criteria. Archives of Neurology 1989;46(8):878-84. - PubMed
Briellmann 1998
-
- Briellmann RS, Nydegger UE, Sturzenegger M, Fierz L, Hess CW, Hauser SP. Long term treatment of chronic inflammatory demyelinating polyradiculoneuropathy: combinations of corticosteroids, plasma exchange, and intravenous immunoglobulin. European Neurology 1998;39(3):190-1. - PubMed
Bright 2014
Broers 2019
Bus 2021
-
- Bus SR, Wieske L, Keddie S, Schaik IN, Eftimov F. Subcutaneous immunoglobulin for chronic inflammatory demyelinating polyradiculoneuropathy. Cochrane Database of Systematic Reviews 2021, Issue 10. Art. No: CD014542. [DOI: 10.1002/14651858.CD014542] - DOI
Chiò 2007
Cornblath 1991
-
- Cornblath DR, Chaudhry V, Griffin JW. Treatment of chronic inflammatory demyelinating polyneuropathy with intravenous immunoglobulin. Annals of Neurology 1991;30(1):104-6. - PubMed
Créange 2009
-
- Créange A, Debouverie M, Jaillon-Rivière V, Taithe F, Liban D, Moutereau A, et al. Home administration of intravenous methylprednisolone for multiple sclerosis relapses: the experience of French multiple sclerosis networks. Multiple Sclerosis 2009;15(9):1085-91. [DOI: 10.1177/1352458509106710] [PMID: ] - DOI - PubMed
de Haan 1993
-
- Haan R, Aaronson N, Limburg M, Hewer RL, Crevel H. Measuring quality of life in stroke. Stroke 1993;24(2):320-7. - PubMed
Deeks 2022
-
- Deeks JJ, Higgins JP, Altman DG, editor(s). Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (updated February 2022). Cochrane, 2022. Available from training.cochrane.org/handbook/archive/v6.3.
Donaghy 1985
-
- Donaghy M, Earl CJ. Ocular palsy preceding chronic relapsing polyneuropathy by several weeks. Annals of Neurology 1985;17(1):49-50. - PubMed
Duhem 1994
Dyck 1982
-
- Dyck PJ, O'Brien P, Oviatt KF. Prednisone improves chronic inflammatory demyelinating polyradiculoneuropathy more than no treatment. Annals of Neurology 1982;11(2):136-41. - PubMed
Dyck 1986
-
- Dyck PJ, Daube J, O'Brien P, Pineda A, Low PA, Windebank AJ, et al. Plasma exchange in chronic inflammatory demyelinating polyradiculoneuropathy. New England Journal of Medicine 1986;314(8):461-5. - PubMed
Dyck 1992
-
- Dyck PJ, Karnes JL, O'Brien PC, Litchy WJ, Low PA, Melton LJ. The Rochester diabetic neuropathy study: reassessment of tests and criteria for diagnosis and staged severity. Neurology 1992;42(6):1164-70. - PubMed
Dyck 1993
-
- Dyck PJ, Prineas JW, Pollard JD. Chronic inflammatory demyelinating polyradiculoneuropathy. In: Thomas PK, Griffin J, Low PA, editors, editors(s). Peripheral Neuropathy. Philadelphia: W.B. Saunders Company, 1993:1498-17.
Elbourne 2002
-
- Elbourne DR, Altman DG, Higgins JP, Curtin F, Wothington HV, Vail A. Meta-analyses involving cross-over trials: methodological issues. International Journal of Epidemiology 2002;31(1):140-9. - PubMed
EUCTR2012‐001996‐34
-
- EUCTR2012-001996-34. A comparative, double-blind, randomised, multicentre efficacy and safety study of ClairYg® versus Tégéline® in maintenance treatment of chronic inflammatory demyelinating polyradiculoneuropathy (CIDP) CNO - CN-01848101. trialsearch.who.int/Trial2.aspx?TrialID=EUCTR2012-001996-34-FR (first received 3 September 2012).
Faed 1989
-
- Faed JM, Day B, Pollock M, Taylor PK, Nukada H, Hammond-Tooke GD. High-dose intravenous human immunoglobulin in chronic inflammatory demyelinating polyneuropathy. Neurology 1989;39(3):422-5. - PubMed
Feasby 1992
-
- Feasby TE. Inflammatory demyelinating polyneuropathies. Neurologic Clinics 1992;10(3):651-70. - PubMed
Follmann 1992
-
- Follmann D, Elliott P, Suh I, Cutler J. Variance imputation for overviews of clinical trials with continuous response. Journal of Clinical Epidemiology 1992;45(7):769-73. - PubMed
Gaebel 2010
GBS study group 1985
-
- The Guillain-Barré Syndrome Study Group. Plasmapheresis and acute Guillain-Barré syndrome. Neurology 1985;35(8):1096-104. - PubMed
Gross 1981
-
- Gross ML, Thomas PK. The treatment of chronic relapsing and chronic progressive idiopathic inflammatory polyneuropathy by plasma exchange. Journal of the Neurological Sciences 1981;52(1):69-78. - PubMed
Guillain‐Barré Syndrome Study Group 1985
-
- The Guillain-Barré Syndrome Study Group. Plasmapheresis and acute Guillain–Barré syndrome. Neurology 1985;35:1096-104. - PubMed
Hahn 1998
-
- Hahn AF. Treatment of chronic inflammatory demyelinating polyneuropathy with intravenous immunoglobulin. Neurology 1998;51(6 Suppl 5):16-21. - PubMed
Higgins 2011
-
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1.
Higgins 2017
-
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0 (updated March 2017). The Cochrane Collaboration, 2017. Available from training.cochrane.org/handbook/archive/v5.2.
Higgins 2022a
-
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (updated February 2022). Cochrane, 2022. Available from www.training.cochrane.org/handbook/archive/v6.3. - PMC - PubMed
Higgins 2022b
-
- Higgins JP, Li T, Deeks JJ, editor(s). Chapter 6: Choosing effect measures and computing estimates of effect. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (updated February 2022). Cochrane, 2022. Available from www.training.cochrane.org/handbook/archive/v6.3.
Higgins 2022c
-
- Higgins J, Lasserson T, Chandler J, Tovey D, Thomas J, Flemyng E, Churchill R. Methodological Expectations of Cochrane Intervention Reviews (MECIR). Standards for the conduct and reporting of new Cochrane Intervention Reviews, reporting of protocols and the planning, conduct and reporting of updates. Version February 2022. Cochrane, 2022. Available at community.cochrane.org/sites/default/files/uploads/MECIR%20Version%20Feb....
Kapoor 2020
Kazatchkine 2001
-
- Kazatchkine MD, Kaveri SV. Immunomodulation of autoimmune and inflammatory disease with intravenous immune globulin. New England Journal of Medicine 2001;345(10):747-55. - PubMed
Latov 2010
-
- Latov N, Deng C, Dalakas MC, Bril V, Donofrio P, Hanna K, et al. Timing and course of clinical response to intravenous immunoglobulin in chronic inflammatory demyelinating polyradiculoneuropathy. Archives of Neurology 2010;67(7):802-7. - PubMed
Laughlin 2009
Lindenbaum 2001
-
- Lindenbaum Y, Kissel JT, Mendell JR. Treatment approaches for Guillain-Barré syndrome and chronic inflammatory demyelinating polyradiculoneuropathy. Neurologic Clinics 2001;19(1):187-204. - PubMed
Lopate 2005
-
- Lopate G, Pestronk A, Al-Lozi M. Treatment of chronic inflammatory demyelinating polyneuropathy with high-dose intermittent intravenous methylprednisolone. Archives of Neurology 2005;62(2):249-54. - PubMed
Lunn 1999
Lunn 2016
-
- Lunn MP, Ellis L, Hadden RD, Rajabally YA, Winer JB, Reilly MM. A proposed dosing algorithm for the individualized dosing of human immunoglobulin in chronic inflammatory neuropathies. Journal of the Peripheral Nervous System 2016;21(1):33-7. - PubMed
Mahdi‐Rogers 2013
-
- Mahdi-Rogers M, Swan AV, Doorn PA, Hughes RA. Immunomodulatory treatment other than corticosteroids, immunoglobulin and plasma exchange for chronic inflammatory demyelinating polyradiculoneuropathy. Cochrane Database of Systematic Reviews 2013, Issue 6. Art. No: CD003280. [DOI: 10.1002/14651858.CD003280.pub4] - DOI - PubMed
Martin 2000
-
- Martin TD. Safety and tolerability of intravenous immunoglobulins. In: Said G, editors(s). Treatment of neurological disorders with intravenous immunoglobulins. London: Martin Duntiz, 2000:181-91.
Matsumuro 1994
-
- Matsumuro K, Izumo S, Umehara F, Osame M. Chronic inflammatory demyelinating polyneuropathy: histological and immunopathological studies on biopsied sural nerves. Journal of the Neurological Sciences 1994;127(2):170-8. - PubMed
McCrone 2003
-
- McCrone P, Chisholm D, Knapp M, Hughes R, Comi G, et al. Cost-utility analysis of intravenous immunoglobulin and prednisolone for chronic inflammatory demyelinating polyradiculoneuropathy. European Journal of Neurology 2003;10(6):687-94. - PubMed
McLeod 1999
-
- McLeod JG, Pollard JD, Macaskill P, Mohamed A, Spring P, Khurana V. Prevalence of chronic inflammatory demyelinating polyneuropathy in New South Wales, Australia. Annals of Neurology 1999;46(6):910-3. - PubMed
Mehndiratta 2012
Merkies 2000
-
- Merkies IS, Schmitz PI, Meche FG, Doorn PA. Psychometric evaluation of a new sensory scale in immune-mediated polyneuropathies. Inflammatory Neuropathy Cause and Treatment (INCAT) Group. Neurology 2000;54(4):943-9. - PubMed
Merkies 2019
Molenaar 1998
Muley 2008
-
- Muley SA, Kelkar P, Parry GJ. Treatment of chronic inflammatory demyelinating polyneuropathy with pulsed oral steroids. Archives of Neurology 2008;65(11):1460-4. - PubMed
Nobile‐Orazio 2015
-
- Nobile-Orazio E, Cocito D, Jann S, Uncini A, Messina P, Antonini G, et al. Frequency and time to relapse after discontinuing 6-month therapy with IVIg or pulsed methylprednisolone in CIDP. Journal of Neurology, Neurosurgery & Psychiatry 2015;86(7):729-34. - PubMed
Pollard 1987
-
- Pollard JD. A critical review of therapies in acute and chronic inflammatory demyelinating polyneuropathies. Muscle & Nerve 1987;10(3):214-21. - PubMed
Rajabally 2006
-
- Rajabally YA, Seow H, Wilson P. Dose of intravenous immunoglobulins in chronic inflammatory demyelinating polyneuropathy. Journal of the Peripheral Nervous System 2006;11(4):325-9. - PubMed
RevMan Web 2022 [Computer program]
-
- Review Manager Web (RevMan Web). Version 4.12.0. The Cochrane Collaboration, 2022. Available at revman.cochrane.org.
RMC Trial Group 2009
-
- RMC Trial Group. Randomised controlled trial of methotrexate for chronic inflammatory demyelinating polyradiculoneuropathy (RMC trial): a pilot, multicentre study. Lancet Neurology 2009;8(2):158-64. - PubMed
Ruts 2005
-
- Ruts L, Koningsveld R, Doorn PA. Distinguishing acute-onset CIDP from Guillain-Barre syndrome with treatment related fluctuations. Neurology 2005;65(1):138-40. - PubMed
Ruts 2010
-
- Ruts L, Drenthen J, Jacobs BC, Doorn PA, Dutch GBS Study Group. Distinguishing acute-onset CIDP from fluctuating Guillain-Barre syndrome: a prospective study. Neurology 2010;74(21):1680-6. - PubMed
Schünemann 2022
-
- Schünemann HJ, Higgins JP, Vist GE, Glasziou P, Akl EA, Skoetz N, et al. Chapter 14: Completing 'Summary of findings' tables and grading the certainty of the evidence. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (updated February 2022). Cochrane, 2022. Available from training.cochrane.org/handbook/archive/v6.3.
Scott 1982
-
- Scott OM, Hyde SA, Goddard C, Dubowitz V. Quantitation of muscle function in children: a prospective study in Duchenne muscular dystrophy. Muscle & Nerve 1982;5(4):291-301. - PubMed
Server 1979
-
- Server AC, Lefkowith J, Braine H, McKhann GM. Treatment of chronic relapsing inflammatory polyradiculoneuropathy by plasma exchange. Annals of Neurology 1979;6(3):258-61. - PubMed
Simmons 1993
-
- Simmons Z, Albers JW, Bromberg MB, Feldman EL. Presentation and initial clinical course in patients with chronic inflammatory demyelinating polyradiculoneuropathy: Comparison of patients without and with monoclonal gammopathy. Neurology 1993;43(11):2202-9. - PubMed
Stiehm 1996
-
- Stiehm ER. Immunoglobulin therapy in primary antibody deficiency and HIV infection. In: Kazatchkine MD, Morell A, editors(s). Intravenous immunoglobulin: research and therapy. 1 edition. Pearl River NY: Parthenon Publishing, 1996:193-203.
van den Bergh 2010
-
- Van den Bergh PY, Hadden RD, Bouche P, Cornblath DR, Hahn A, Illa I, et al. European Federation of Neurological Societies/Peripheral Nerve Society guideline on management of chronic inflammatory demyelinating polyradiculoneuropathy: report of a joint task force of the European Federation of Neurological Societies and the Peripheral Nerve Society - first revision. European Journal of Neurology 2010;17(3):356-63. - PubMed
van den Bergh 2021
-
- Van den Bergh PYK, Doorn PA, Hadden RDM, Avau B, Vankrunkelsven P, Allen JA, et al. European Academy of Neurology/Peripheral Nerve Society guideline on diagnosis and treatment of chronic inflammatory demyelinating polyradiculoneuropathy: Report of a joint Task Force-Second revision. European Journal of Neurology 2021;28(11):3556-83. - PubMed
van der Meché 1989
-
- Meché FG, Vermeulen M, Busch HF. Chronic inflammatory demyelinating polyneuropathy. Brain 1989;112(Pt 6):1563-71. - PubMed
van der Meché 1997
-
- Meché FG, Doorn PA. Future developments in the treatment of immune-related polyneuropathies. European Neurology 1997;38(3):230-7. - PubMed
van Doorn 1990a
-
- Doorn PA, Rossi F, Brand A, Lint M, Vermeulen M, Kazatchkine MD. On the mechanism of high-dose intravenous immunoglobulin treatment of patients with chronic inflammatory demyelinating polyneuropathy. Journal of Neuroimmunology 1990;29(1-3):57-64. - PubMed
van Doorn 1991
-
- Doorn PA, Vermeulen M, Brand A, Mulder PG, Busch HF. Intravenous immunoglobulin treatment in patients with chronic inflammatory demyelinating polyneuropathy. Archives of Neurology 1991;48(2):217-20. - PubMed
van Nes 2008
-
- Nes SI, Faber CG, Merkies IS. Outcome measure in immune-mediated neuropathies: the need to standardize their use and to understand the clinimetric essentials. Journal of the Peripheral Nervous System 2008;13(2):136-47. - PubMed
van Schaik 1994
van Schaik 2010
-
- Schaik IN, Eftimov F, Doorn PA, Brusse E, den Berg LH, Pol WL, et al. Pulsed high-dose dexamethasone versus standard prednisolone treatment for chronic inflammatory demyelinating polyradiculoneuropathy (PREDICT study): a double-blind, randomised, controlled trial. Lancet Neurology 2010;9(3):245-53. - PubMed
van Schaik 2018
-
- Schaik IN, Bril V, Geloven N, Hartung HP, Lewis RA, Sobue G, et al. Subcutaneous immunoglobulin for maintenance treatment in chronic inflammatory demyelinating polyneuropathy (PATH): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Neurology 2018;17(1):35-46. - PubMed
Vermeulen 1985
-
- Vermeulen M, Meché FG, Speelman JD, Weber A, Busch HF. Plasma and gamma-globulin infusion in chronic inflammatory polyneuropathy. Journal of the Neurological Sciences 1985;70(3):317-26. - PubMed
WHO 1982
Yu 1999
-
- Yu Z, Lennon VA. Mechanism of intravenous immune globulin therapy in antibody-mediated autoimmune diseases. New England Journal of Medicine 1999;340(3):227-8. - PubMed
References to other published versions of this review
Eftimov 2013
Van Schaik 1999
Van Schaik 2002
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

