Hybrid Ginseng-derived Extracellular Vesicles-Like Particles with Autologous Tumor Cell Membrane for Personalized Vaccination to Inhibit Tumor Recurrence and Metastasis
- PMID: 38353384
- PMCID: PMC11077655
- DOI: 10.1002/advs.202308235
Hybrid Ginseng-derived Extracellular Vesicles-Like Particles with Autologous Tumor Cell Membrane for Personalized Vaccination to Inhibit Tumor Recurrence and Metastasis
Abstract
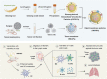
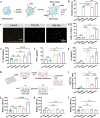
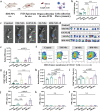
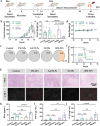
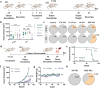
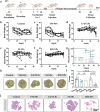
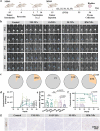
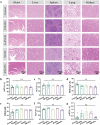
Personalized cancer vaccines based on resected tumors from patients is promising to address tumor heterogeneity to inhibit tumor recurrence or metastasis. However, it remains challenge to elicit immune activation due to the weak immunogenicity of autologous tumor antigens. Here, a hybrid membrane cancer vaccine is successfully constructed by membrane fusion to enhance adaptive immune response and amplify personalized immunotherapy, which formed a codelivery system for autologous tumor antigens and immune adjuvants. Briefly, the functional hybrid vesicles (HM-NPs) are formed by hybridizing ginseng-derived extracellular vesicles-like particles (G-EVLPs) with the membrane originated from the resected autologous tumors. The introduction of G-EVLPs can enhance the phagocytosis of autologous tumor antigens by dendritic cells (DCs) and facilitate DCs maturation through TLR4, ultimately activating tumor-specific cytotoxic T lymphocytes (CTLs). HM-NPs can indeed strengthen specific immune responses to suppress tumors recurrence and metastasis including subcutaneous tumors and orthotopic tumors. Furthermore, a long-term immune protection can be obtained after vaccinating with HM-NPs, and prolonging the survival of animals. Overall, this personalized hybrid autologous tumor vaccine based on G-EVLPs provides the possibility of mitigating tumor recurrence and metastasis after surgery while maintaining good biocompatibility.
Keywords: ginseng‐derived nanoparticles; hybrid nanoparticles; long‐term protection; mature DCs; personalized tumor vaccine.
© 2024 The Authors. Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- a) Liu C., Liu X., Xiang X., Pang X., Chen S., Zhang Y., Ren E., Zhang L., Liu X., Lv P., Nat. Nanotechnol. 2022, 17, 531; - PubMed
- b) Blass E., Ott P. A., Nat. Rev. Clin. Oncol. 2021, 18, 215; - PMC - PubMed
- c) Fan Q., Ma Q., Bai J., Xu J., Fei Z., Dong Z., Maruyama A., Leong K. W., Liu Z., Wang C., Sci. Adv. 2020, 6, eabb4639; - PMC - PubMed
- d) Lin L., Pei Y., Li Z., Luo D., Interdiscip. Med. 2023, 1, e20220008.
-
- a) Mota I., Patrucco E., Mastini C., Mahadevan N. R., Thai T. C., Bergaggio E., Cheong T.‐C., Leonardi G., Karaca‐Atabay E., Campisi M., Nat. Cancer 2023, 4, 1016; - PMC - PubMed
- b) Wang C., Steinmetz N. F., Adv. Funct. Mater. 2020, 30, 2002299; - PMC - PubMed
- c) Nejo T., Yamamichi A., Almeida N. D., Goretsky Y. E., Okada H., Semin. Immunol. 2020, 47, 101385. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- CXZX202225/Jiangsu Provincial Medical Innovation Center
- Jiangsu Province's Outstanding Leader Program of Traditional Chinese Medicine
- XPT82204292/Fundamental Research Funds of Nanjing Universities of Chinese Medicine
- BK20220472/Natural Science Foundation of Jiangsu Province
- 82204292/National Natural Science Foundation of China
LinkOut - more resources
Full Text Sources
