Modulation of Cerebrospinal Fluid Dysregulation via a SPAK and OSR1 Targeted Framework Nucleic Acid in Hydrocephalus
- PMID: 38353402
- PMCID: PMC11077654
- DOI: 10.1002/advs.202306622
Modulation of Cerebrospinal Fluid Dysregulation via a SPAK and OSR1 Targeted Framework Nucleic Acid in Hydrocephalus
Abstract
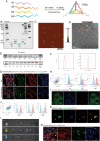
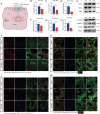
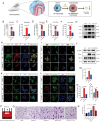
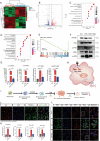
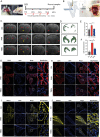
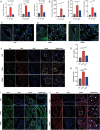
Hydrocephalus is one of the most common brain disorders and a life-long incurable condition. An empirical "one-size-fits-all" approach of cerebrospinal fluid (CSF) shunting remains the mainstay of hydrocephalus treatment and effective pharmacotherapy options are currently lacking. Macrophage-mediated ChP inflammation and CSF hypersecretion have recently been identified as a significant discovery in the pathogenesis of hydrocephalus. In this study, a pioneering DNA nano-drug (TSOs) is developed by modifying S2 ssDNA and S4 ssDNA with SPAK ASO and OSR1 ASO in tetrahedral framework nucleic acids (tFNAs) and synthesis via a one-pot annealing procedure. This construct can significantly knockdown the expression of SPAK and OSR1, along with their downstream ion channel proteins in ChP epithelial cells, thereby leading to a decrease in CSF secretion. Moreover, these findings indicate that TSOs effectively inhibit the M0 to M1 phenotypic switch of ChP macrophages via the MAPK pathways, thus mitigating the cytokine storm. In in vivo post-hemorrhagic hydrocephalus (PHH) models, TSOs significantly reduce CSF secretion rates, alleviate ChP inflammation, and prevent the onset of hydrocephalus. These compelling results highlight the potential of TSOs as a promising therapeutic option for managing hydrocephalus, with significant applications in the future.
Keywords: DNA nanomaterials; cerebrospinal fluid hypersecretion; choroid plexus; cytokines; hydrocephalus; macrophages.
© 2024 The Authors. Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Kahle K. T., Kulkarni A. V., D. D. Limbrick, Jr , Warf B. C., Lancet 2016, 387, 788. - PubMed
-
- Zaksaite T., Loveday C., Edginton T., Spiers H. J., Smith A. D., Cortex 2023, 160, 67. - PubMed
-
- McAllister J. P., Williams M. A., Walker M. L., Kestle J. R. W., Relkin N. R., Anderson A. M., Gross P. H., Browd S. R., J. Neurosurg. 2015, 123, 1427. - PubMed
-
- Kulkarni A. V., Cochrane D. D., McNeely P. D., J. Pediatr. 2008, 153, 689. - PubMed
-
- Kahle K. T., Kulkarni A. V., Limbrick D. D., Warf B. C., Lancet 2016, 387, 788. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 2019YFA0110600/National Key R&D Program of China
- 82201501/National Natural Science Foundation of China
- 82370929/National Natural Science Foundation of China
- 81970916/National Natural Science Foundation of China
- 2022NSFSC0002/Sichuan Science and Technology Program
- 22ZDYF2619/Sichuan Science and Technology Program
- 2022JDTD0021/Sichuan Province Youth Science and Technology Innovation Team
- RD03202302/Research and Develop Program, West China Hospital of Stomatology Sichuan University
- 2023NSFC1581/Natural Science Foundation of Sichuan Province
- 2020HXFH013/1·3·5 project for disciplines of excellence-Clinical Research Incubation Project, West China Hospital, Sichuan University
LinkOut - more resources
Full Text Sources
Medical
