Enhancing CAR Macrophage Efferocytosis Via Surface Engineered Lipid Nanoparticles Targeting LXR Signaling
- PMID: 38353580
- PMCID: PMC11081841
- DOI: 10.1002/adma.202308377
Enhancing CAR Macrophage Efferocytosis Via Surface Engineered Lipid Nanoparticles Targeting LXR Signaling
Abstract
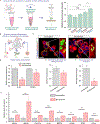
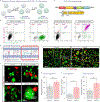
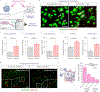
The removal of dying cells, or efferocytosis, is an indispensable part of resolving inflammation. However, the inflammatory microenvironment of the atherosclerotic plaque frequently affects the biology of both apoptotic cells and resident phagocytes, rendering efferocytosis dysfunctional. To overcome this problem, a chimeric antigen receptor (CAR) macrophage that can target and engulf phagocytosis-resistant apoptotic cells expressing CD47 is developed. In both normal and inflammatory circumstances, CAR macrophages exhibit activity equivalent to antibody blockage. The surface of CAR macrophages is modified with reactive oxygen species (ROS)-responsive therapeutic nanoparticles targeting the liver X receptor pathway to improve their cell effector activities. The combination of CAR and nanoparticle engineering activated lipid efflux pumps enhances cell debris clearance and reduces inflammation. It is further suggested that the undifferentiated CAR-Ms can transmigrate within a mico-fabricated vessel system. It is also shown that our CAR macrophage can act as a chimeric switch receptor (CSR) to withstand the immunosuppressive inflammatory environment. The developed platform has the potential to contribute to the advancement of next-generation cardiovascular disease therapies and further studies include in vivo experiments.
Keywords: CAR macrophage; atherosclerosis; efferocytosis; lipid nanoparticle; β‐cyclodextrin.
© 2024 The Authors. Advanced Materials published by Wiley‐VCH GmbH.
Figures



References
-
- a)Chao MP, Majeti R, Weissman IL, Nature Reviews Cancer 2012, 12, 58; - PubMed
- b)Feng M, Marjon KD, Zhu F, Weissman-Tsukamoto R, Levett A, Sullivan K, Kao KS, Markovic M, Bump PA, Jackson HM, Choi TS, Chen J, Banuelos AM, Liu J, Gip P, Cheng L, Wang D, Weissman IL, Nature Communications 2018, 9, 3194. - PMC - PubMed
-
- Voll RE, Herrmann M, Roth EA, Stach C, Kalden JR, Girkontaite I, Nature 1997, 390, 350. - PubMed
-
- Stocker R, Keaney JF Jr., Physiol Rev 2004, 84, 1381. - PubMed
-
- a)Duewell P, Kono H, Rayner KJ, Sirois CM, Vladimer G, Bauernfeind FG, Abela GS, Franchi L, Nuñez G, Schnurr M, Espevik T, Lien E, Fitzgerald KA, Rock KL, Moore KJ, Wright SD, Hornung V, Latz E, Nature 2010, 464, 1357; - PMC - PubMed
- b)Rajamäki K, Lappalainen J, Oörni K, Välimäki E, Matikainen S, Kovanen PT, Eklund KK, PLoS One 2010, 5, e11765. - PMC - PubMed
MeSH terms
Substances
Grants and funding
- P30ES005022/NH/NIH HHS/United States
- NHLBI/HL/NHLBI NIH HHS/United States
- P30 ES005022/ES/NIEHS NIH HHS/United States
- U01HL150852/HL/NHLBI NIH HHS/United States
- R21 AR071101/AR/NIAMS NIH HHS/United States
- CSCR22ERG023/ALZ/Alzheimer's Association/United States
- R01 NS130836/NS/NINDS NIH HHS/United States
- RM1 NS133003/NS/NINDS NIH HHS/United States
- T32 EB005583/EB/NIBIB NIH HHS/United States
- S10OD026876/NH/NIH HHS/United States
- S10 OD026876/OD/NIH HHS/United States
- R21 NS132556/NS/NINDS NIH HHS/United States
- T32 GM135141/GM/NIGMS NIH HHS/United States
- R01 DC016612/DC/NIDCD NIH HHS/United States
- U01 HL150852/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials

