Association of an In-Hospital Desirability of Outcomes Ranking Scale With Postdischarge Health-Related Quality of Life: A Secondary Analysis of the Life After Pediatric Sepsis Evaluation
- PMID: 38353586
- PMCID: PMC11153013
- DOI: 10.1097/PCC.0000000000003470
Association of an In-Hospital Desirability of Outcomes Ranking Scale With Postdischarge Health-Related Quality of Life: A Secondary Analysis of the Life After Pediatric Sepsis Evaluation
Abstract
Objectives: To develop a desirability of outcome ranking (DOOR) scale for use in children with septic shock and determine its correlation with a decrease in 3-month postadmission health-related quality of life (HRQL) or death.
Design: Secondary analysis of the Life After Pediatric Sepsis Evaluation prospective study.
Setting: Twelve U.S. PICUs, 2013-2017.
Patients: Children (1 mo-18 yr) with septic shock.
Interventions: None.
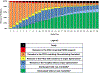
Measurements and main results: We applied a 7-point pediatric critical care (PCC) DOOR scale: 7: death; 6: extracorporeal life support; 5: supported by life-sustaining therapies (continuous renal replacement therapy, vasoactive, or invasive ventilation); 4: hospitalized with or 3: without organ dysfunction; 2: discharged with or 1: without new morbidity to patients by assigning the highest applicable score on specific days post-PICU admission. We analyzed Spearman rank-order correlations (95% CIs) between proximal outcomes (PCC-DOOR scale on days 7, 14, and 21, ventilator-free days, cumulative 28-day Pediatric Logistic Organ Dysfunction-2 (PELOD-2) scores, and PICU-free days) and 3-month decrease in HRQL or death. HRQL was measured by Pediatric Quality of Life Inventory 4.0 or Functional Status II-R for patients with developmental delay. Patients who died were assigned the worst possible HRQL score. PCC-DOOR scores were applied to 385 patients, median age 6 years (interquartile range 2, 13) and 177 (46%) with a complex chronic condition(s). Three-month outcomes were available for 245 patients (64%) and 42 patients (17%) died. PCC-DOOR scale on days 7, 14, and 21 demonstrated fair correlation with the primary outcome (-0.42 [-0.52, -0.31], -0.47 [-0.56, -0.36], and -0.52 [-0.61, -0.42]), similar to the correlations for cumulative 28-day PELOD-2 scores (-0.51 [-0.59, -0.41]), ventilator-free days (0.43 [0.32, 0.53]), and PICU-free days (0.46 [0.35, 0.55]).
Conclusions: The PCC-DOOR scale is a feasible, practical outcome for pediatric sepsis trials and demonstrates fair correlation with decrease in HRQL or death at 3 months.
Copyright © 2024 by the Society of Critical Care Medicine and the World Federation of Pediatric Intensive and Critical Care Societies.
Conflict of interest statement
Dr. Logan’s institution received funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) (K23HD096018) (R01HD073362 and cooperative agreements U10-HD050012, U10-HD050096, U10-HD063108, U10-HD049983 U10-HD049981, U10-HD063114, and U10-HD063106). Drs. Logan, Banks, Reeder, Meert, Zimmerman, and Maddux received support for article research from the National Institutes of Health (NIH). Drs. Banks, Bennett, and Zimmerman’s institutions received funding from the NICHD. Dr. Banks disclosed government work. Drs. Reeder and Meert’s institutions received funding from the NIH. Dr. Bennett’s institution received funding from the National Center for Advancing Translational Sciences and the National Heart, Lung, and Blood Institute. Dr. Zimmerman’s institution received funding from Immunexpress; he received funding from Elsevier Publishing. Dr. Maddux’s institution received funding from the NICHD (K23 HD096018) and the Francis Family Foundation. The remaining authors have disclosed that they do not have any potential conflicts of interest.
Figures
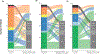
Similar articles
-
Critical Illness Factors Associated With Long-Term Mortality and Health-Related Quality of Life Morbidity Following Community-Acquired Pediatric Septic Shock.Crit Care Med. 2020 Mar;48(3):319-328. doi: 10.1097/CCM.0000000000004122. Crit Care Med. 2020. PMID: 32058369 Free PMC article.
-
Trajectory of Mortality and Health-Related Quality of Life Morbidity Following Community-Acquired Pediatric Septic Shock.Crit Care Med. 2020 Mar;48(3):329-337. doi: 10.1097/CCM.0000000000004123. Crit Care Med. 2020. PMID: 32058370 Free PMC article.
-
Change in Functional Status During Hospital Admission and Long-Term Health-Related Quality of Life Among Pediatric Septic Shock Survivors.Pediatr Crit Care Med. 2023 Dec 1;24(12):e573-e583. doi: 10.1097/PCC.0000000000003312. Epub 2023 Jun 22. Pediatr Crit Care Med. 2023. PMID: 37346003 Free PMC article.
-
Social Determinants of Health and Health-Related Quality of Life Following Pediatric Septic Shock: Secondary Analysis of the Life After Pediatric Sepsis Evaluation Dataset, 2014-2017.Pediatr Crit Care Med. 2024 Sep 1;25(9):804-815. doi: 10.1097/PCC.0000000000003550. Epub 2024 Jun 5. Pediatr Crit Care Med. 2024. PMID: 38836691
-
Predicting functional and quality-of-life outcomes following pediatric sepsis: performance of PRISM-III and PELOD-2.Pediatr Res. 2023 Dec;94(6):1951-1957. doi: 10.1038/s41390-023-02619-w. Epub 2023 Apr 26. Pediatr Res. 2023. PMID: 37185949 Free PMC article.
References
-
- Hartman ME, Linde-Zwirble WT, Angus DC, et al.: Trends in the epidemiology of pediatric severe sepsis. Pediatr Crit Care Med 2013; 14(7):686–693. - PubMed
-
- Merritt C, Menon K, Agus MSD, et al.: Beyond Survival: Pediatric Critical Care Interventional Trial Outcome Measure Preferences of Families and Healthcare Professionals. Pediatr Crit Care Med 2018; 19(2):e105–e111. - PubMed
-
- Ruth A, McCracken CE, Fortenberry JD, et al.: Pediatric severe sepsis: current trends and outcomes from the Pediatric Health Information Systems database. Pediatr Crit Care Med 2014; 15(9):828–838. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

