Increasing maternal glucose concentrations is insufficient to restore placental glucose transfer in chorionic somatomammotropin RNA interference pregnancies
- PMID: 38353640
- PMCID: PMC11376830
- DOI: 10.1152/ajpendo.00331.2023
Increasing maternal glucose concentrations is insufficient to restore placental glucose transfer in chorionic somatomammotropin RNA interference pregnancies
Abstract
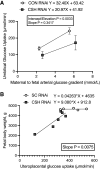
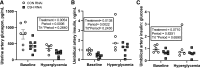
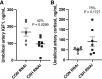
We previously demonstrated impaired placental nutrient transfer in chorionic somatomammotropin (CSH) RNA interference (RNAi) pregnancies, with glucose transfer being the most impacted. Thus, we hypothesized that despite experimentally elevating maternal glucose, diminished umbilical glucose uptake would persist in CSH RNAi pregnancies, demonstrating the necessity of CSH for adequate placental glucose transfer. Trophectoderm of sheep blastocysts (9 days of gestational age; dGA) were infected with a lentivirus expressing either nontargeting control (CON RNAi; n = 5) or CSH-specific shRNA (CSH RNAi; n = 7) before transfer into recipient sheep. At 126 dGA, pregnancies were fitted with vascular catheters and underwent steady-state metabolic studies (3H2O transplacental diffusion) at 137 ± 0 dGA, before and during a maternal hyperglycemic clamp. Umbilical glucose and oxygen uptakes, as well as insulin and IGF1 concentrations, were impaired (P ≤ 0.01) in CSH RNAi fetuses and were not rescued by elevated maternal glucose. This is partially due to impaired uterine and umbilical blood flow (P ≤ 0.01). However, uteroplacental oxygen utilization was greater (P ≤ 0.05) during the maternal hyperglycemic clamp, consistent with greater placental oxidation of substrates. The relationship between umbilical glucose uptake and the maternal-fetal glucose gradient was analyzed, and while the slope (CON RNAi, Y = 29.54X +74.15; CSH RNAi, Y = 19.05X + 52.40) was not different, the y-intercepts and elevation were (P = 0.003), indicating reduced maximal glucose transport during maternal hyperglycemia. Together, these data suggested that CSH plays a key role in modulating placental metabolism that ultimately promotes maximal placental glucose transfer.NEW & NOTEWORTHY The current study demonstrated a novel, critical autocrine role for chorionic somatomammotropin in augmenting placental glucose transfer and maintaining placental oxidative metabolism. In pregnancies with CSH deficiency, excess glucose in maternal circulation is insufficient to overcome fetal hypoglycemia due to impaired placental glucose transfer and elevated placental metabolic demands. This suggests that perturbations in glucose transfer in CSH RNAi pregnancies are due to compromised metabolic efficiency along with reduced placental mass.
Keywords: RNA interference; chorionic somatomammotropin; fetal growth restriction; glucose transfer; placenta.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures





References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

