Sex-related differences in patients presenting with heart failure-related cardiogenic shock
- PMID: 38353681
- PMCID: PMC10954943
- DOI: 10.1007/s00392-024-02392-8
Sex-related differences in patients presenting with heart failure-related cardiogenic shock
Abstract
Background: Heart failure-related cardiogenic shock (HF-CS) accounts for a significant proportion of all CS cases. Nevertheless, there is a lack of evidence on sex-related differences in HF-CS, especially regarding use of treatment and mortality risk in women vs. men. This study aimed to investigate potential differences in clinical presentation, use of treatments, and mortality between women and men with HF-CS.
Methods: In this international observational study, patients with HF-CS (without acute myocardial infarction) from 16 tertiary-care centers in five countries were enrolled between 2010 and 2021. Logistic and Cox regression models were used to assess differences in clinical presentation, use of treatments, and 30-day mortality in women vs. men with HF-CS.
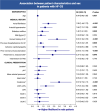
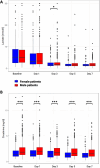
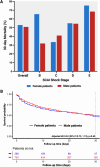
Results: N = 1030 patients with HF-CS were analyzed, of whom 290 (28.2%) were women. Compared to men, women were more likely to be older, less likely to have a known history of heart failure or cardiovascular risk factors, and lower rates of highly depressed left ventricular ejection fraction and renal dysfunction. Nevertheless, CS severity as well as use of treatments were comparable, and female sex was not independently associated with 30-day mortality (53.0% vs. 50.8%; adjusted HR 0.94, 95% CI 0.75-1.19).
Conclusions: In this large HF-CS registry, sex disparities in risk factors and clinical presentation were observed. Despite these differences, the use of treatments was comparable, and both sexes exhibited similarly high mortality rates. Further research is necessary to evaluate if sex-tailored treatment, accounting for the differences in cardiovascular risk factors and clinical presentation, might improve outcomes in HF-CS.
Keywords: Cardiogenic shock; Gender differences; Heart failure; Sex disparities; Women in heart failure.
© 2024. The Author(s).
Conflict of interest statement
B.S. reports speaker fees from Abiomed, Abbott, and AstraZeneca, outside of the submitted work. L.F.B. reports speaker fees from Abiomed, outside of the submitted work. S.B. reports grants and personal fees from Abbott Diagnostics, Bayer, Siemens, and Thermo Fisher; grants from Singulex; and personal fees from Abbott, AstraZeneca, Amgen, Medtronic, Pfizer, Roche, Siemens Diagnostics, and Novartis, outside of the submitted work. J.D. received speaker fees from AstraZeneca, Boehringer Ingelheim, and Bayer. J.S. received travel grants from Abiomed, outside the submitted work. D.E. reports speaker fees from Abiomed, Biotronik, and Daiichi sankyo, outside of the submitted work. P.K. was partially supported by European Union AFFECT-AF (grant agreement 847770), and MAESTRIA (grant agreement 965286), British Heart Foundation (PG/17/30/32961; PG/20/22/35093; AA/18/2/34218), German Centre for Cardiovascular Research supported by the German Ministry of Education and Research (DZHK), Deutsche Forschungsgemeinschaft (Ki 509167694), and Leducq Foundation; receives research support for basic, translational, and clinical research projects from European Union, British Heart Foundation, Leducq Foundation, Medical Research Council (UK), and German Centre for Cardiovascular Research, from several drug and device companies active in atrial fibrillation, and has received honoraria from several such companies in the past, but not in the last 3 years; and is listed as inventor on two issued patents held by University of Hamburg (Atrial Fibrillation Therapy WO 2015140571, Markers for Atrial Fibrillation WO 2016012783). S.K. received research support from Cytosorbents and Daiichi Sankyo. He also received lecture fees from ADVITOS, Biotest, Daiichi Sankyo, Fresenius Medical Care, Gilead, Mitsubishi Tanabe Pharma, MSD, Pfizer, Shionogi, and Zoll. He received consultant fees from Fresenius, Gilead, MSD, and Pfizer. P.L. received speaker honoraria and consulting fees from AstraZeneca, Bayer, Pfizer, and Edwards Lifesciences, and research honoraria from Edwards Lifesciences, outside of the submitted work. S.M.-W. reports Abiomed unrestricted grant for JenaMacs trial. Speakers honoraria: Abiomed, Boston Scientific, Pfizer, Daichi Sankyo, outside of the submitted work. N. M. has received personal fees from Edwards Lifesciences, Medtronic, Biotronik, Novartis, Sanofi Genzyme, AstraZeneca, Pfizer, Bayer, Abbott, Abiomed, and Boston Scientific outside the submitted work. M.O. reports speaker honoraria and travel compensations from companies Abbott Medical, AstraZeneca, Abiomed, Bayer vital, BIOTRONIK, Bristol-Myers Squibb, CytoSorbents, Daiichi Sankyo Deutschland, Edwards Lifesciences Services, and Sedana Medical. F.P. consultant for Abiomed. T.R. received speaker honoraria and consulting fees from AstraZeneca, Bayer, Pfizer, and Daiichi Sankyo. None of them was related to this study. C.S. reports lecture fees from Abiomed, outside the submitted work. P.C.S. received honoraria for lectures/consulting from Novartis, Vifor, Bayer, Pfizer, Boehringer Ingelheim, AstraZeneca, Cardior, BMS, Abiomed, Pharmacosmos, and Amgen not related to this article; and research support for the department from Boehringer Ingelheim, Edwards, and Abiomed not related to this article. D.W. reports speaker fees from Abiomed, AstraZeneca, Bayer, Berlin-Chemie, Boehringer Ingelheim, Novartis, and Medtronic, outside of the submitted work. E.B.W. reports grants from Boehringer Ingelheim, and personal fees from Amarin, Amgen, AstraZeneca, Daiichi Sankyo, Bayer, Boehringer Ingelheim, CVRx, and Novartis outside the submitted work. All other authors did not declare a conflict of interest.
Figures

References
-
- McDonagh TA, Metra M, Adamo M, et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Developed by the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2021;42(36):3599–3726. doi: 10.1093/EURHEARTJ/EHAB368. - DOI - PubMed
-
- Baran DA, Grines CL, Bailey S, et al. SCAI clinical expert consensus statement on the classification of cardiogenic shock: this document was endorsed by the American College of Cardiology (ACC), the American Heart Association (AHA), the Society of Critical Care Medicine (SCCM), and the Societ. Catheter Cardiovasc Interv. 2019;94(1):29–37. doi: 10.1002/ccd.28329. - DOI - PubMed
-
- Naidu SS, Baran DA, Jentzer JC, et al. SCAI SHOCK stage classification expert consensus update: a review and incorporation of validation studies: this statement was endorsed by the American College of Cardiology (ACC), American College of Emergency Physicians (ACEP), American Heart Association. J Am Coll Cardiol. 2022;79(9):933–946. doi: 10.1016/j.jacc.2022.01.018. - DOI - PubMed
-
- Berg DD, Bohula EA, van Diepen S, Katz JN, Alviar CL, Baird-Zars VM, Barnett CF, Barsness GW, Burke JA, Cremer PC, Cruz J, Daniels LB, DeFilippis AP, Haleem A, Hollenberg SM, Horowitz JM, Keller N, Kontos MC, Lawler PR, Menon V, ... Morrow DA (2019) Epidemiology of shock in contemporary cardiac intensive care units. Circ Cardiovasc Qual Outcomes 12(3):e005618. 10.1161/CIRCOUTCOMES.119.005618 - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

