Bioactive potential of Bio-C Temp demonstrated by systemic mineralization markers and immunoexpression of bone proteins in the rat connective tissue
- PMID: 38353838
- PMCID: PMC10867037
- DOI: 10.1007/s10856-024-06781-3
Bioactive potential of Bio-C Temp demonstrated by systemic mineralization markers and immunoexpression of bone proteins in the rat connective tissue
Abstract
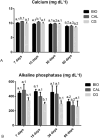
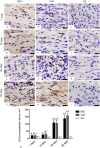
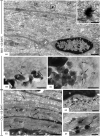
Intracanal medications are used in endodontic treatment due to their antibacterial activity and ability to induce the periapical repair. Among the intracanal medications, the Calen (CAL; SS. White, Brazil) is a calcium hydroxide-based medication that provides an alkaline pH and releases calcium, exerting an antimicrobial activity. Bio-C Temp (BIO; Angelus, Brazil), a ready-to-use bioceramic intracanal medication, was designed to stimulate the mineralized tissues formation. Here, we investigated the bioactive potential of BIO in comparison to the CAL in the rat subcutaneous. Polyethylene tubes filled with medications, and empty tubes (control group, CG) were implanted in the subcutaneous tissue of rats. After 7, 15, 30 and 60 days, the blood was collected for calcium (Ca+2) and alkaline phosphatase (ALP) measurement, and the capsules around the implants were processed for morphological analyses. The data were submitted to two-way ANOVA and Tukey test (p < 0.05). At 7, 15 and 30 days, the ALP level was grater in BIO and CAL than in CG (p < 0.0001). At 7 and 15 days, greater Ca+2 level was seen in the serum of CAL samples. From 7 to 60 days, an increase in the number of fibroblasts, osteocalcin- and osteopontin-immunolabelled cells was observed in BIO and CAL groups (p < 0.0001). In all periods, BIO and CAL specimens showed von Kossa-positive structures. Moreover, ultrastructural analysis revealed globules of mineralization in the capsules around the BIO and CAL specimens. Thus Bio-C Temp caused an increase in the ALP, osteocalcin and osteopontin, which may have allowed the formation of calcite, suggesting bioactive potential.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Mohammadi Z, Dummer PM. Properties and applications of calcium hydroxide in endodontics and dental traumatology. Int Endod J. 2011;44:697–730. 10.1111/j.1365-2591.2011.01886 - PubMed
-
- Estrela C, Sydney GB, Bammann LL, Felippe-Júnior O. Mechanism of action of calcium and hydroxyl ions of calcium hydroxide on tissue and bacteria. Braz Dent J. 1995;6:85–90. - PubMed
-
- Mizuno M, Banzai Y. Calcium ion release from calcium hydroxide stimulated fibronectin gene expression in dental pulp cells and the differentiation of dental pulp cells to mineralized tissue forming cells by fibronectin. Int Endod J. 2008;41:933–8. 10.1111/j.1365-2591.2008.01420.x - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

