Anti-CD38 targeted nanotrojan horses stimulated by acoustic waves as therapeutic nanotools selectively against Burkitt's lymphoma cells
- PMID: 38353903
- PMCID: PMC10866835
- DOI: 10.1186/s11671-024-03976-z
Anti-CD38 targeted nanotrojan horses stimulated by acoustic waves as therapeutic nanotools selectively against Burkitt's lymphoma cells
Abstract
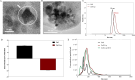
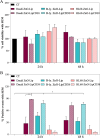
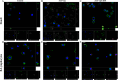
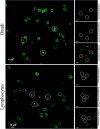
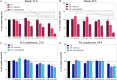
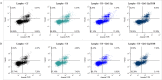
The horizon of nanomedicine research is moving toward the design of therapeutic tools able to be completely safe per se, and simultaneously be capable of becoming toxic when externally activated by stimuli of different nature. Among all the stimuli, ultrasounds come to the fore as an innovative approach to produce cytotoxicity on demand in presence of NPs, without invasiveness, with high biosafety and low cost. In this context, zinc oxide nanoparticles (NPs) are among the most promising metal oxide materials for theranostic application due to their optical and semi-conductor properties, high surface reactivity, and their response to ultrasound irradiation. Here, ZnO nanocrystals constitute the stimuli-responsive core with a customized biomimicking lipidic shielding, resembling the composition of natural extracellular vesicles. This core-shell hybrid structure provides high bio- and hemocompatibility towards healthy cells and is here proofed for the treatment of Burkitt's Lymphoma. This is a very common haematological tumor, typically found in children, for which consolidated therapies are so far the combination of chemo-therapy drugs and targeted immunotherapy. In this work, the proposed safe-by-design antiCD38-targeted hybrid nanosystem exhibits an efficient selectivity toward cancerous cells, and an on-demand activation, leading to a significant killing efficacy due to the synergistic interaction between US and targeted hybrid NPs. Interestingly, this innovative treatment does not significantly affect healthy B lymphocytes nor a negative control cancer cell line, a CD38- acute myeloid leukemia, being thus highly specific and targeted. Different characterization and analyses confirmed indeed the effective formation of targeted hybrid ZnO NPs, their cellular internalization and the damages produced in Burkitt's Lymphoma cells only with respect to the other cell lines. The presented work holds promises for future clinical applications, as well as translation to other tumor types.
Keywords: Biomimetic nanoparticles; Monoclonal antibody fragments; Stimuli-responsive nanoparticles; Targeted therapy; Ultrasounds; Zinc oxide.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




Similar articles
-
Iron-Doped ZnO Nanoparticles as Multifunctional Nanoplatforms for Theranostics.Nanomaterials (Basel). 2021 Oct 6;11(10):2628. doi: 10.3390/nano11102628. Nanomaterials (Basel). 2021. PMID: 34685064 Free PMC article.
-
Nanotechnological engineering of extracellular vesicles for the development of actively targeted hybrid nanodevices.Cell Biosci. 2022 May 14;12(1):61. doi: 10.1186/s13578-022-00784-9. Cell Biosci. 2022. PMID: 35568919 Free PMC article.
-
A comparative analysis of low intensity ultrasound effects on living cells: from simulation to experiments.Biomed Microdevices. 2022 Oct 24;24(4):35. doi: 10.1007/s10544-022-00635-x. Biomed Microdevices. 2022. PMID: 36279001 Free PMC article.
-
Theranostic Nanoparticles for RNA-Based Cancer Treatment.Acc Chem Res. 2019 Jun 18;52(6):1496-1506. doi: 10.1021/acs.accounts.9b00101. Epub 2019 May 28. Acc Chem Res. 2019. PMID: 31135134 Free PMC article. Review.
-
Burkitt's lymphoma: clinicopathologic features and differential diagnosis.Oncologist. 2006 Apr;11(4):375-83. doi: 10.1634/theoncologist.11-4-375. Oncologist. 2006. PMID: 16614233 Review.
Cited by
-
Functional nanocrystal as effective contrast agents for dual-mode imaging: Live-cell sonoluminescence and contrast-enhanced echography.Ultrason Sonochem. 2025 Feb;113:107242. doi: 10.1016/j.ultsonch.2025.107242. Epub 2025 Jan 22. Ultrason Sonochem. 2025. PMID: 39874777 Free PMC article.
-
Diving on the Surface of a Functional Metal Oxide through a Multiscale Exploration of Drug-Nanocrystal Interactions.ACS Appl Mater Interfaces. 2025 Feb 19;17(7):10432-10445. doi: 10.1021/acsami.4c19916. Epub 2025 Feb 10. ACS Appl Mater Interfaces. 2025. PMID: 39930563 Free PMC article.
-
Acoustically Driven Hybrid Nanocrystals for In Vivo Pancreatic Cancer Treatment.ACS Appl Mater Interfaces. 2025 Feb 26;17(8):11873-11887. doi: 10.1021/acsami.4c21975. Epub 2025 Feb 17. ACS Appl Mater Interfaces. 2025. PMID: 39960802 Free PMC article.
-
Ultrasound-assisted water oxidation: unveiling the role of piezoelectric metal-oxide sonocatalysts for cancer treatment.Biomed Microdevices. 2024 Aug 19;26(3):37. doi: 10.1007/s10544-024-00720-3. Biomed Microdevices. 2024. PMID: 39160324 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
