O-RADS MRI risk stratification system: pearls and pitfalls
- PMID: 38353905
- PMCID: PMC10866854
- DOI: 10.1186/s13244-023-01577-5
O-RADS MRI risk stratification system: pearls and pitfalls
Abstract
In 2021, the American College of Radiology (ACR) Ovarian-Adnexal Reporting and Data System (O-RADS) MRI Committee developed a risk stratification system and lexicon for assessing adnexal lesions using MRI. Like the BI-RADS classification, O-RADS MRI provides a standardized language for communication between radiologists and clinicians. It is essential for radiologists to be familiar with the O-RADS algorithmic approach to avoid misclassifications. Training, like that offered by International Ovarian Tumor Analysis (IOTA), is essential to ensure accurate and consistent application of the O-RADS MRI system. Tools such as the O-RADS MRI calculator aim to ensure an algorithmic approach. This review highlights the key teaching points, pearls, and pitfalls when using the O-RADS MRI risk stratification system.Critical relevance statement This article highlights the pearls and pitfalls of using the O-RADS MRI scoring system in clinical practice.Key points• Solid tissue is described as displaying post- contrast enhancement.• Endosalpingeal folds, fimbriated end of the tube, smooth wall, or septa are not solid tissue.• Low-risk TIC has no shoulder or plateau. An intermediate-risk TIC has a shoulder and plateau, though the shoulder is less steep compared to outer myometrium.
Keywords: MRI; O-RADS; Ovarian lesion; Stratification.
© 2024. The Author(s).
Conflict of interest statement
YL consults for Calyx Clinical Trial Solutions. The remaining authors declare that they have no competing interests.
Figures
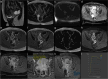
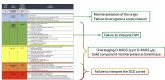
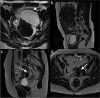
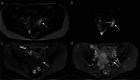

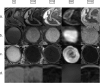
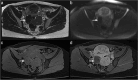
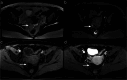
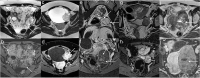
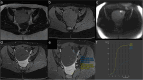
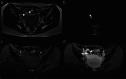
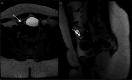
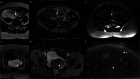
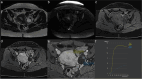
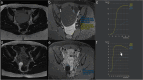

Similar articles
-
Practical Tips for Reporting Adnexal Lesions Using O-RADS MRI.Radiographics. 2023 Jul;43(7):e220142. doi: 10.1148/rg.220142. Radiographics. 2023. PMID: 37319025
-
O-RADS for Ultrasound: A User's Guide, From the AJR Special Series on Radiology Reporting and Data Systems.AJR Am J Roentgenol. 2021 May;216(5):1150-1165. doi: 10.2214/AJR.20.25064. Epub 2020 Dec 23. AJR Am J Roentgenol. 2021. PMID: 33355485 Review.
-
O-RADS US Risk Stratification and Management System: A Consensus Guideline from the ACR Ovarian-Adnexal Reporting and Data System Committee.Radiology. 2020 Jan;294(1):168-185. doi: 10.1148/radiol.2019191150. Epub 2019 Nov 5. Radiology. 2020. PMID: 31687921
-
O-RADS MRI Risk Stratification System: Guide for Assessing Adnexal Lesions from the ACR O-RADS Committee.Radiology. 2022 Apr;303(1):35-47. doi: 10.1148/radiol.204371. Epub 2022 Jan 18. Radiology. 2022. PMID: 35040672 Free PMC article. Review.
-
O-RADS MRI Classification of Indeterminate Adnexal Lesions: Time-Intensity Curve Analysis Is Better Than Visual Assessment.Radiology. 2022 Jun;303(3):566-575. doi: 10.1148/radiol.210342. Epub 2022 Mar 1. Radiology. 2022. PMID: 35230183
Cited by
-
Navigating Ovarian-Adnexal Reporting and Data System Magnetic Resonance Imaging (O-RADS MRI): A Review of Its Evolution, Current Advances, and Persistent Challenges in Ovarian Imaging.Cureus. 2025 Jun 25;17(6):e86717. doi: 10.7759/cureus.86717. eCollection 2025 Jun. Cureus. 2025. PMID: 40718198 Free PMC article. Review.
-
European Society of Urogenital Radiology (ESUR) guidelines on MR imaging prior to fertility-sparing treatments in patients with cervical, endometrial, and ovarian cancers.Eur Radiol. 2025 May 13. doi: 10.1007/s00330-025-11625-x. Online ahead of print. Eur Radiol. 2025. PMID: 40358675
References
-
- Rizzo S, Cozzi A, Dolciami M, et al (2023) O-RADS MRI: A Systematic Review and Meta-Analysis of Diagnostic Performance and Category-wise Malignancy Rates. Radiology 307(1):e220795. 10.1148/radiol.220795. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

