Implementation of Best Practices in Pancreatic Cancer Care in the Netherlands: A Stepped-Wedge Randomized Clinical Trial
- PMID: 38353966
- PMCID: PMC10867778
- DOI: 10.1001/jamasurg.2023.7872
Implementation of Best Practices in Pancreatic Cancer Care in the Netherlands: A Stepped-Wedge Randomized Clinical Trial
Abstract
Importance: Implementation of new cancer treatment strategies as recommended by evidence-based guidelines is often slow and suboptimal.
Objective: To improve the implementation of guideline-based best practices in the Netherlands in pancreatic cancer care and assess the impact on survival.
Design, setting, and participants: This multicenter, stepped-wedge cluster randomized trial compared enhanced implementation of best practices with usual care in consecutive patients with all stages of pancreatic cancer. It took place from May 22, 2018 through July 9, 2020. Data were analyzed from April 1, 2022, through February 1, 2023. It included all patients in the Netherlands with pathologically or clinically diagnosed pancreatic ductal adenocarcinoma. This study reports 1-year follow-up (or shorter in case of deceased patients).
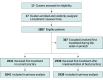
Intervention: The 5 best practices included optimal use of perioperative chemotherapy, palliative chemotherapy, pancreatic enzyme replacement therapy (PERT), referral to a dietician, and use of metal stents in patients with biliary obstruction. A 6-week implementation period was completed, in a randomized order, in all 17 Dutch networks for pancreatic cancer care.
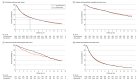
Main outcomes and measures: The primary outcome was 1-year survival. Secondary outcomes included adherence to best practices and quality of life (European Organisation for Research and Treatment of Cancer [EORTC] global health score).
Results: Overall, 5887 patients with pancreatic cancer (median age, 72.0 [IQR, 64.0-79.0] years; 50% female) were enrolled, 2641 before and 2939 after implementation of best practices (307 during wash-in period). One-year survival was 24% vs 23% (hazard ratio, 0.98, 95% CI, 0.88-1.08). There was no difference in the use of neoadjuvant chemotherapy (11% vs 11%), adjuvant chemotherapy (48% vs 51%), and referral to a dietician (59% vs 63%), while the use of palliative chemotherapy (24% vs 30%; odds ratio [OR], 1.38; 95% CI, 1.10-1.74), PERT (34% vs 45%; OR, 1.64; 95% CI, 1.28-2.11), and metal biliary stents increased (74% vs 83%; OR, 1.78; 95% CI, 1.13-2.80). The EORTC global health score did not improve (area under the curve, 43.9 vs 42.8; median difference, -1.09, 95% CI, -3.05 to 0.94).
Conclusions and relevance: In this randomized clinical trial, implementation of 5 best practices in pancreatic cancer care did not improve 1-year survival and quality of life. The finding that most patients received no tumor-directed treatment paired with the poor survival highlights the need for more personalized treatment options.
Trial registration: ClinicalTrials.gov Identifier: NCT03513705.
Conflict of interest statement
Figures
Comment in
-
Stepped-wedge clinical trials in pancreatic cancer: a step backward in improving overall survival or a leap forward to enhance quality of care?Gland Surg. 2025 Feb 28;14(2):263-267. doi: 10.21037/gs-24-472. Epub 2025 Feb 25. Gland Surg. 2025. PMID: 40115846 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous