Two-pore channels regulate endomembrane tension to enable remodeling and resolution of phagolysosomes
- PMID: 38354262
- PMCID: PMC10895354
- DOI: 10.1073/pnas.2309465121
Two-pore channels regulate endomembrane tension to enable remodeling and resolution of phagolysosomes
Abstract
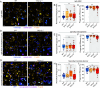
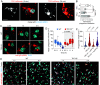
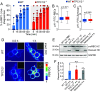
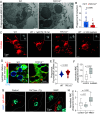
Phagocytes promptly resolve ingested targets to replenish lysosomes and maintain their responsiveness. The resolution process requires that degradative hydrolases, solute transporters, and proteins involved in lipid traffic are delivered and made active in phagolysosomes. It also involves extensive membrane remodeling. We report that cation channels that localize to phagolysosomes were essential for resolution. Specifically, the conductance of Na+ by two-pore channels (TPCs) and the presence of a Na+ gradient between the phagolysosome lumen and the cytosol were critical for the controlled release of membrane tension that permits deformation of the limiting phagolysosome membrane. In turn, membrane deformation was a necessary step to efficiently transport the cholesterol extracted from cellular targets, permeabilizing them to hydrolases. These results place TPCs as regulators of endomembrane remodeling events that precede target degradation in cases when the target is bound by a cholesterol-containing membrane. The findings may help to explain lipid metabolism dysfunction and autophagic flux impairment reported in TPC KO mice and establish stepwise regulation to the resolution process that begins with lysis of the target.
Keywords: glycocalyx; phagocytosis; solute transport.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

