Complement Activation Is Associated With Disease Severity in Multiple Sclerosis
- PMID: 38354323
- PMCID: PMC10913171
- DOI: 10.1212/NXI.0000000000200212
Complement Activation Is Associated With Disease Severity in Multiple Sclerosis
Erratum in
-
Complement Activation Is Associated With Disease Severity in Multiple Sclerosis.Neurol Neuroimmunol Neuroinflamm. 2024 May;11(3):e200246. doi: 10.1212/NXI.0000000000200246. Epub 2024 Mar 19. Neurol Neuroimmunol Neuroinflamm. 2024. PMID: 38507658 Free PMC article. No abstract available.
Abstract
Background and objectives: Histopathologic studies have identified immunoglobulin (Ig) deposition and complement activation as contributors of CNS tissue damage in multiple sclerosis (MS). Intrathecal IgM synthesis is associated with higher MS disease activity and severity, and IgM is the strongest complement-activating immunoglobulin. In this study, we investigated whether complement components (CCs) and complement activation products (CAPs) are increased in persons with MS, especially in those with an intrathecal IgM synthesis, and whether they are associated with disease severity and progression.
Methods: CC and CAP levels were quantified in plasma and CSF of 112 patients with clinically isolated syndrome (CIS), 127 patients with MS (90 relapsing-remitting, 14 primary progressive, and 23 secondary progressive), 31 inflammatory neurologic disease, and 44 symptomatic controls from the Basel CSF databank study. Patients with CIS/MS were followed in the Swiss MS cohort study (median 6.3 years). Levels of CC/CAP between diagnosis groups were compared; in CIS/MS, associations of CC/CAP levels with intrathecal Ig synthesis, baseline Expanded Disability Status Scale (EDSS) scores, MS Severity Score (MSSS), and neurofilament light chain (NfL) levels were investigated by linear regression, adjusted for age, sex, and albumin quotient.
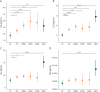
Results: CSF (but not plasma) levels of C3a, C4a, Ba, and Bb were increased in patients with CIS/MS, being most pronounced in those with an additional intrathecal IgM production. In CIS, doubling of C3a and C4a in CSF was associated with 0.31 (CI 0.06-0.56; p = 0.016) and 0.32 (0.02-0.62; p = 0.041) increased EDSS scores at lumbar puncture. Similarly, doubling of C3a and Ba in CIS/MS was associated with 0.61 (0.19-1.03; p < 0.01) and 0.74 (0.18-1.31; p = 0.016) increased future MSSS. In CIS/MS, CSF levels of C3a, C4a, Ba, and Bb were associated with increased CSF NfL levels, e.g., doubling of C3a was associated with an increase of 58% (Est. 1.58; CI 1.37-1.81; p < 0.0001).
Discussion: CNS-compartmentalized activation of the classical and alternative pathways of complement is increased in CIS/MS and associated with the presence of an intrathecal IgM production. Increased complement activation within the CSF correlates with EDSS, future MSSS, and NfL levels, supporting the concept that complement activation contributes to MS pathology and disease progression. Complement inhibition should be explored as therapeutic target to attenuate disease severity and progression in MS.
Conflict of interest statement
K. Stein, S.A. Schaedelin, A.M. Maceski, A. Orleth, S. Meier, E. Willemse, I. Heijnen, A. Regeniter, P. Benkert, M. Limberg, and B. Fischer-Barnicol report no conflicts of interest. J. Oechtering received research support from the Swiss MS Society and served on advisory boards for Roche and Merck. F. Qureshi is an employee of Octave Bioscience, Inc. T. Derfuss received speaker fees, research support, travel support, and/or served on Advisory Boards, data safety monitoring boards, or Steering Committees of Actelion, Alexion, Celgene, Polyneuron, Novartis Pharma, Merck Serono, Biogen, Teva, Bayer-Schering, GeNeuro, Mitsubishi Pharma, MedDay, Roche, and Genzyme. M. D'Souza has received travel support from Bayer AG, Biogen, Teva Pharmaceuticals, and Sanofi Genzyme and research support from the University Hospital Basel. L. Achtnichts served on scientific advisory boards for Celgene, Novartis Pharmaceuticals, Merck, Biogen, Sanofi Genzyme, Roche, and Bayer; received funding for travel and/or speaker honoraria from Celgene, Biogen, Sanofi Genzyme, Novartis, Merck Serono, Roche, Teva, and the Swiss MS Society; and research support from Biogen, Sanofi, Genzyme, and Novartis. S. Mueller received speaker fees, research support, travel support, and/or served on advisory boards by Almirall, Alexion, Bayer, Biogen, Bristol Myers Squibb, Celgene, Genzyme, Merck-Serono, Teva, Novartis, and Roche. A. Salmen received speaker honoraria for activities with Bristol Myers Squibb, CSL Behring, Novartis, and Roche and research support from the Baasch Medicus Foundation, the Medical Faculty of the University of Bern, and the Swiss MS Society. P.H. Lalive received honoraria for speaking and/or travel expenses from Biogen, Merck, Novartis, and Roche; consulting fees from Biogen, GeNeuro, Merck, Novartis, Roche; research support from Biogen, Merck, and Novartis. None were related to this work. C. Bridel served on scientific advisory boards for Biogen, Novartis, and BMS. C. Pot received consulting fees and/or travel compensation, used exclusively for research support, for activities with Biogen, Merck, Novartis, Roche, and Sanofi Genzyme. R.A. Du Pasquier has served on scientific advisory boards for Biogen, Celgene, Merck, Novartis, Roche, and Sanofi. He has received funding for travel or speaker honoraria from Roche. H. Wiendl receives honoraria for acting as a member of Scientific Advisory Boards for Janssen, Merck, and Novartis as well as speaker honoraria and travel support from Alexion, Amicus Therapeuticus, Biogen, Biologix, Bristol Myers Squibb, Cognomed, F. Hoffmann-La Roche Ltd., Gemeinnützige Hertie-Stiftung, Medison, Merck, Novartis, Roche Pharma AG, Genzyme, TEVA, and WebMD Global. Prof. Wiendl is acting as a paid consultant for Biogen, Bristol Myers Squibb, EMD Serono, Idorsia, Immunic, Novartis, Roche, Sanofi, the Swiss MS Society, and UCB. His research is funded by the German Ministry for Education and Research (BMBF), Deutsche Forschungsgesellschaft (DFG), Deutsche Myasthenie Gesellschaft e.V., Alexion, Amicus Therapeutics Inc., Argenx, Biogen, CSL Behring, F. Hoffmann-La Roche, Genzyme, Merck KgaA, Novartis Pharma, Roche Pharma, and UCB Biopharma. C. Gobbi: The University Hospital Basel (USB), as an employer of Cristina Granziera, has received the following fees which were used exclusively for research support: (1) advisory board and consultancy fees from Actelion, Novartis, Genzyme, and F. Hoffmann-La Roche; (2) speaker fees from Biogen and Genzyme-Sanofi; and (3) research support from F. Hoffmann-La Roche Ltd. Before her employment at USB, she has also received speaker honoraria and travel funding from Novartis. L. Kappos 's: employer (University Hospital Basel), has received and dedicated to research support steering committee, advisory board, and consultancy fees (Abbvie, Actelion, Almirall, Auriga Vison AG, Bayer HealthCare, Biogen, Eisai, EMD Derono Inc, Genzyme, Genentech Inc, F. Hoffmann-La Roche, Japan Tobacco, Janssen Pharmaceuticals Inc, Merck, Minoryx Therapeutics SL, Novartis, Sanofi, Santhera, Senda Biosciences, Shionogi BV, and TG Therapeutics); speaker fees (Bayer HealthCare, Biogen, Celgene, Genzyme, Janssen Pharmaceuticals Inc, Merck, Novartis, Roche, and Sanofi); support of educational activities (Allergan, Bayer HealthCare, Biogen, CSL Behring, Genzyme, Merck, Novartis, Roche, Pfizer, Sanofi, Shire, and Teva); license fees for Neurostatus products; and grants (Bayer HealthCare, Biogen, European Union, Innosuisse, Merck, Novartis, Roche Research Foundation, Swiss MS Society, and Swiss National Research Foundation). M. Trendelenburg has research collaborations with Roche, Novartis, and Idorsia (all Switzerland). D. Leppert is a Chief Medical Officer of GeNeuro. J.D. Luenemann received speaker fees, research support, travel support, and/or served on advisory boards by Abbvie, Alexion, Argenx, Biogen, Merck, Novartis, Roche, Sanofi, and Takeda. J. Kuhle received speaker fees, research support, travel support, and/or served on advisory boards by Swiss MS Society, Swiss National Research Foundation (320030_189140/1), University of Basel, Progressive MS Alliance, Bayer, Biogen, Bristol Myers Squibb, Celgene, Merck, Novartis, Octave Bioscience, Roche, and Sanofi. Go to
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
