Circulating and Imaging Biomarkers of Radium-223 Response in Metastatic Castration-Resistant Prostate Cancer
- PMID: 38354328
- PMCID: PMC11556836
- DOI: 10.1200/PO.23.00230
Circulating and Imaging Biomarkers of Radium-223 Response in Metastatic Castration-Resistant Prostate Cancer
Abstract
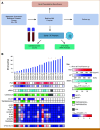
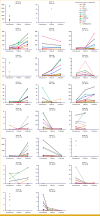
Purpose: Radium-223 improves overall survival (OS) and reduces skeletal events in patients with bone metastatic castration-resistant prostate cancer (CRPC), but relevant biomarkers are lacking. We evaluated automated bone scan index (aBSI) and circulating tumor cell (CTC) analyses as potential biomarkers of prognosis and activity.
Patients and methods: Patients with bone metastatic CRPC were enrolled on a prospective single-arm study of standard radium-223. 99mTc-MDP bone scan images at baseline, 2 months, and 6 months were quantitated using aBSI. CTCs at baseline, 1 month, and 2 months were enumerated and assessed for RNA expression of prostate cancer-specific genes using microfluidic enrichment followed by droplet digital polymerase chain reaction.
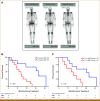
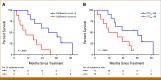
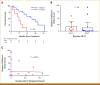
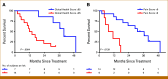
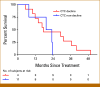
Results: The median OS was 21.3 months in 22 patients. Lower baseline aBSI and minimal change in aBSI (<+0.7) from baseline to 2 months were each associated with better OS (P = .00341 and P = .0139, respectively). The higher baseline CTC count of ≥5 CTC/7.5 mL was associated with worse OS (median, 10.1 v 32.9 months; P = .00568). CTCs declined at 2 months in four of 15 patients with detectable baseline CTCs. Among individual genes in CTCs, baseline expression of the splice variant AR-V7 was significantly associated with worse OS (hazard ratio, 5.20 [95% CI, 1.657 to 16.31]; P = .00195). Baseline detectable AR-V7, higher aBSI, and CTC count ≥5 CTC/7.5 mL continued to have a significant independent negative impact on OS after controlling for prostate-specific antigen or alkaline phosphatase.
Conclusion: Quantitative bone scan assessment with aBSI and CTC analyses are prognostic markers in patients treated with radium-223. AR-V7 expression in CTCs is a particularly promising prognostic biomarker and warrants validation in larger cohorts.
Conflict of interest statement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (
No other potential conflicts of interest were reported.
Figures

References
-
- Parker C, Nilsson S, Heinrich D, et al. : Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med 369:213-223, 2013 - PubMed
-
- Sartor O, Coleman R, Nilsson S, et al. : Effect of radium-223 dichloride on symptomatic skeletal events in patients with castration-resistant prostate cancer and bone metastases: Results from a phase 3, double-blind, randomised trial. Lancet Oncol 15:738-746, 2014 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

