Feasibility of Diffusion-weighted Imaging (DWI) for Assessing Cerebrospinal Fluid Dynamics: DWI-fluidography in the Brains of Healthy Subjects
- PMID: 38355106
- PMCID: PMC11996255
- DOI: 10.2463/mrms.mp.2022-0152
Feasibility of Diffusion-weighted Imaging (DWI) for Assessing Cerebrospinal Fluid Dynamics: DWI-fluidography in the Brains of Healthy Subjects
Abstract
Purpose: The present study aimed to investigate whether diffusion-weighted imaging (DWI) can qualify and quantify cerebrospinal fluid (CSF) dynamics in the brains of healthy subjects. For this purpose, we developed new DWI-based fluidography and compared the CSF dynamics seen on the fluidography with two apparent diffusion coefficients obtained with different DWI signal models at anatomical spaces filled by CSF.
Methods: DWI with multiple b values was performed for 10 subjects using a 7T MRI scanner. DWI-fluidography based on the DWI signal variations in different motion probing gradient directions was developed for visualizing the CSF dynamics voxel-by-voxel. DWI signals were measured using an ROI in the representative CSF-filled anatomical spaces in the brain. For the multiple DWI signals, the mono-exponential and kurtosis models were fitted and two kinds of apparent diffusion coefficients (ADCC and ADCK) were estimated in each space using the Gaussian and non-Gaussian diffusion models, respectively.
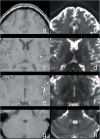
Results: DWI-fluidography could qualitatively represent the features of CSF dynamics in each anatomical space. ADCs indicated that the motions at the foramen of Monro, the cistern of the velum interpositum, the quadrigeminal cistern, the Sylvian cisterns, and the fourth ventricle were more drastic than those at the subarachnoid space and anterior horns of the lateral ventricle. Those results seen in ADCs were identical to the findings on DWI-fluidography.
Conclusion: DWI-fluidography based on the features of DWI signals could show differences of CSF dynamics among anatomical spaces.
Keywords: cerebrospinal fluid; diffusion-weighted imaging; magnetic resonance imaging; neurofluid.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures



Similar articles
-
Can low b value diffusion weighted imaging evaluate the character of cerebrospinal fluid dynamics?Jpn J Radiol. 2019 Feb;37(2):135-144. doi: 10.1007/s11604-018-0790-8. Epub 2018 Nov 8. Jpn J Radiol. 2019. PMID: 30406868
-
Diffusion analysis of fluid dynamics with incremental strength of motion proving gradient (DANDYISM) to evaluate cerebrospinal fluid dynamics.Jpn J Radiol. 2021 Apr;39(4):315-323. doi: 10.1007/s11604-020-01075-4. Epub 2021 Jan 2. Jpn J Radiol. 2021. PMID: 33389526 Free PMC article.
-
Low b-value Diffusion Tensor Imaging to Analyze the Dynamics of Cerebrospinal Fluid: Resolving Intravoxel Pseudorandom Motion into Ordered and Disordered Motions.Magn Reson Med Sci. 2025 Jan 1;24(1):46-57. doi: 10.2463/mrms.mp.2023-0081. Epub 2023 Oct 27. Magn Reson Med Sci. 2025. PMID: 37899254 Free PMC article.
-
Diffusion magnetic resonance imaging of cerebrospinal fluid dynamics: Current techniques and future advancements.NMR Biomed. 2024 Sep;37(9):e5162. doi: 10.1002/nbm.5162. Epub 2024 May 7. NMR Biomed. 2024. PMID: 38715420 Free PMC article. Review.
-
Visualizing non-Gaussian diffusion: clinical application of q-space imaging and diffusional kurtosis imaging of the brain and spine.Magn Reson Med Sci. 2012;11(4):221-33. doi: 10.2463/mrms.11.221. Magn Reson Med Sci. 2012. PMID: 23269009 Review.
Cited by
-
Diffusion-derived intravoxel-incoherent motion anisotropy relates to CSF and blood flow.Magn Reson Med. 2025 Mar;93(3):930-941. doi: 10.1002/mrm.30294. Epub 2024 Nov 6. Magn Reson Med. 2025. PMID: 39503237 Free PMC article.
References
-
- Le Bihan D, Breton E, Lallemand D, Grenier P, Cabanis E, Laval-Jeantet M. MR imaging of intravoxel incoherent motions: Application to diffusion and perfusion in neurologic disorders. Radiology 1986; 161:401–407. - PubMed
-
- Le Bihan D, Breton E, Lallemand D, Aubin ML, Vignaud J, Laval-Jeantet M. Separation of diffusion and perfusion in intravoxel incoherent motion MR imaging. Radiology 1988; 168:497–505. - PubMed
-
- Le Bihan D, Turner R, Douek P, Patronas N. Diffusion MR imaging: Clinical applications. AJR Am J Roentgenol 1992; 159:591–599. - PubMed
-
- Le Bihan D, Turner R, Moonen CT, Pekar J. Imaging of diffusion and microcirculation with gradient sensitization: Design, strategy, and significance. J Magn Reson Imaging 1991; 1:7–28. - PubMed
-
- Einstein A. Investigations on the theory of the Browniaan movement. New York: Dover Publications. 1956.

