Patient symptoms, self-management, and unscheduled healthcare use during the first 6 months of targeted oral anticancer agent therapy: protocol for a mixed-methods US study
- PMID: 38355181
- PMCID: PMC10868296
- DOI: 10.1136/bmjopen-2023-081375
Patient symptoms, self-management, and unscheduled healthcare use during the first 6 months of targeted oral anticancer agent therapy: protocol for a mixed-methods US study
Abstract
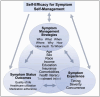
Introduction: Targeted oral anticancer agents (OAAs) are increasingly used to treat cancer, including haematological malignancies and ovarian cancer, but they can cause serious symptomatic side effects such as arrhythmias, hypertension, and hyperglycaemia. Unaddressed OAA symptoms or inadequately managed symptoms may also lead to unnecessary and unscheduled healthcare use that decreases patient quality of life and financially burdens both patients and the healthcare system. Limited information is available about patient symptoms, self-management behaviours, and use of healthcare services over time while taking targeted OAAs, but is needed to ensure successful OAA therapy. The primary objective is to understand patient experiences and behaviours on initiating targeted OAA, and elicit cancer care clinicians' (ie, physicians, advanced practice practitioners, nurses, and pharmacists) perspectives on supporting patients during therapy. Study results will inform comprehensive and realistic interventions that minimise disruptions to therapy while maximising quality of life.
Methods and analysis: We will conduct a remote single-arm, convergent-parallel mixed-methods cohort study within a large academic medical centre. A minimum of 60 patients will be enrolled. Patients will complete several validated patient-reported outcome measures at six timepoints over 6 months. Mixed-effects logistic regression will be used to predict the primary binary outcome of unscheduled healthcare use by patient self-efficacy for symptom self-management. Semistructured interviews will be conducted with patients and clinicians and thematically analysed. Triangulated quantitative and qualitative results will be reported using cross-case comparison joint display.
Ethics and dissemination: This study protocol is approved by the Institutional Review Board of University of Michigan Medical School (IRBMED). Study results will be published in peer-reviewed journals, presented at conferences, and disseminated to study participants.
Keywords: adverse events; health services; oncology.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: KF had funding from AstraZeneca to understand a model of collaboration between primary care and oncology pharmacists in managing patients with new oral anticancer agents. The funded study did not have any relationship with the current protocol.
Figures
Similar articles
-
Families' healthcare experiences for children with inherited metabolic diseases: protocol for a mixed methods cohort study.BMJ Open. 2022 Feb 22;12(2):e055664. doi: 10.1136/bmjopen-2021-055664. BMJ Open. 2022. PMID: 35193919 Free PMC article.
-
Cost-effectiveness of the ONCORAL multidisciplinary programme for the management of outpatients taking oral anticancer agents at risk of drug-related event: protocol for a pragmatic randomised controlled study.BMJ Open. 2024 Feb 17;14(2):e074956. doi: 10.1136/bmjopen-2023-074956. BMJ Open. 2024. PMID: 38367968 Free PMC article.
-
Protocol for a type 2 hybrid effectiveness-implementation study expanding, implementing and evaluating electronic health record-integrated patient-reported symptom monitoring in a multisite cancer centre.BMJ Open. 2022 May 3;12(5):e059563. doi: 10.1136/bmjopen-2021-059563. BMJ Open. 2022. PMID: 35504641 Free PMC article.
-
A Concept Analysis of Oral Anticancer Agent Self-management.Cancer Nurs. 2022 Mar-Apr 01;45(2):E374-E387. doi: 10.1097/NCC.0000000000000934. Cancer Nurs. 2022. PMID: 33654013 Free PMC article. Review.
-
Behavioural modification interventions for medically unexplained symptoms in primary care: systematic reviews and economic evaluation.Health Technol Assess. 2020 Sep;24(46):1-490. doi: 10.3310/hta24460. Health Technol Assess. 2020. PMID: 32975190 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources