Chronic Treatment with Serotonin Selective Reuptake Inhibitors Does Not Affect Regrowth of Serotonin Axons Following Amphetamine Injury in the Mouse Forebrain
- PMID: 38355299
- PMCID: PMC10867722
- DOI: 10.1523/ENEURO.0444-22.2023
Chronic Treatment with Serotonin Selective Reuptake Inhibitors Does Not Affect Regrowth of Serotonin Axons Following Amphetamine Injury in the Mouse Forebrain
Abstract
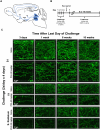
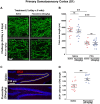
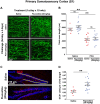
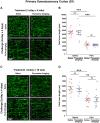
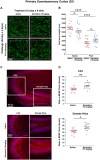
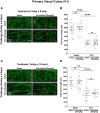
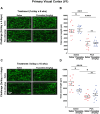
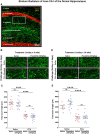
A current hypothesis to explain the limited recovery following brain and spinal cord trauma stems from the dogma that neurons in the mammalian central nervous system lack the ability to regenerate their axons after injury. Serotonin (5-HT) neurons in the adult brain are a notable exception in that they can slowly regrow their axons following chemical or mechanical lesions. This process of regrowth occurs without intervention over several months and results in anatomical recovery that approximates the preinjured state. During development, serotonin is a trophic factor, playing a role in both cell survival and axon growth. Additionally, some studies have shown that stroke patients treated after injury with serotonin selective reuptake inhibitors (SSRIs) appeared to have improved recovery. To test the hypothesis that serotonin can influence the regrowth of 5-HT axons, mice received a high dose of para-chloroamphetamine (PCA) to induce widespread retrograde degeneration of 5-HT axons. Then, after a short rest period to avoid any interaction with the acute injury phase, SSRIs were administered daily for 6 or 10 weeks. Using immunohistochemistry in 5-HT transporter-GFP BAC transgenic mice, we determined that while PCA led to a rapid initial decrease in total 5-HT axon length in the somatosensory cortex, visual cortex, or area CA1 of the hippocampus, treatment with either fluoxetine or sertraline (two different SSRIs) did not affect the recovery of axon length. These results suggest that chronic SSRI treatment does not affect the regrowth of 5-HT axons and argue against SSRIs as a potential therapy following brain injury.
Keywords: brain injury; fluoxetine; regeneration; serotonin; serotonin selective reuptake inhibitors; sertraline.
Copyright © 2024 Janowitz and Linden.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

References
-
- Alamri F, al Shoyaib A, Syeara N, Paul A, Jayaraman S, Karamyan S, Arumugam T, Karamyan V (2021) Delayed atomoxetine or fluoxetine treatment coupled with limited voluntary running promotes motor recovery in mice after ischemic stroke. Neural Regen Res 16:1244–1251. 10.4103/1673-5374.301031 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
