Are the results of open randomised controlled trials comparing antipsychotic drugs in schizophrenia biased? Exploratory meta- and subgroup analysis
- PMID: 38355616
- PMCID: PMC10866997
- DOI: 10.1038/s41537-024-00442-8
Are the results of open randomised controlled trials comparing antipsychotic drugs in schizophrenia biased? Exploratory meta- and subgroup analysis
Abstract
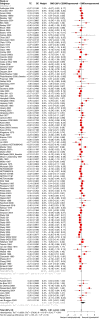
A recent meta-epidemiological study did not reveal major differences between the results of blinded and open randomised-controlled trials (RCTs). Fewer patients may consent to double-blind RCTs than to open RCTs, compromising generalisability, making this question very important. However, the issue has not been addressed in schizophrenia. We used a database of randomised, acute-phase antipsychotic drug trials. Whenever at least one open and one blinded RCT was available for a comparison of two drugs, we contrasted the results by random-effects meta-analysis with subgroup tests. The primary outcome was overall symptoms as measured by the Positive and Negative Syndrome Scale, supplemented by seven secondary efficacy and side-effect outcomes. We also examined whether open RCTs were biased in favour of more recently introduced antipsychotics, less efficacious or more prone to side-effects antipsychotics, and pharmaceutical sponsors. 183 RCTs (155 blinded and 28 open) with 34715 participants comparing two active drugs were available. The results did not suggest general differences between open and blinded RCTs, which examined two active drugs. Only 12 out of 122 subgroup tests had a p-value below 0.1, four below 0.05, and if a Bonferroni correction for multiple tests had been applied, only one would have been significant. There were some exceptions which, however, did not always confirm the originally hypothesized direction of bias. Due to the relatively small number of open RCTs, our analysis is exploratory, but this fundamental question should be given more scientific attention. Currently, open RCTs should be excluded from meta-analyses, at least in sensitivity analyses.
© 2024. The Author(s).
Conflict of interest statement
In the last 3 years, S.L. has received honoraria for advising/consulting and/or for lectures and/or for educational material from Angelini, Boehringer Ingelheim, Eisai, Ekademia, GedeonRichter, Janssen, Karuna, Kynexis, Lundbeck, Medichem, Medscape, Mitsubishi, Otsuka, NovoNordisk, Recordati, Rovi, Teva, A.T. received lecture fees from Sumitomo Dainippon Pharma, Eisai, Janssen Pharmaceutical, Meiji-Seika Pharma, Mitsubishi Tanabe Pharma, Otsuka and Takeda Pharmaceutical. TAF reports personal fees from Boehringer-Ingelheim, DT Axis, Kyoto University Original, Shionogi, SONY and UpToDate, and a grant from Shionogi. In addition, T.A.F. has patents 2020-548587 and 2022-082495 pending and intellectual properties for Kokoro-app licensed to Mitsubishi-Tanabe. The remaining authors declare no competing interests.
Figures
Similar articles
-
An open, large, 6-month naturalistic study of outcome in schizophrenic outpatients, treated with olanzapine.Hum Psychopharmacol. 2011 Jan;26(1):81-5. doi: 10.1002/hup.1173. Epub 2011 Feb 9. Hum Psychopharmacol. 2011. PMID: 23055416 Review.
-
The response of subgroups of patients with schizophrenia to different antipsychotic drugs: a systematic review and meta-analysis.Lancet Psychiatry. 2022 Nov;9(11):884-893. doi: 10.1016/S2215-0366(22)00304-2. Epub 2022 Oct 10. Lancet Psychiatry. 2022. PMID: 36228647
-
[Antipsychotics in bipolar disorders].Encephale. 2004 Sep-Oct;30(5):417-24. doi: 10.1016/s0013-7006(04)95456-5. Encephale. 2004. PMID: 15627046 Review. French.
-
Standard versus reduced dose of antipsychotics for relapse prevention in multi-episode schizophrenia: a systematic review and meta-analysis of randomised controlled trials.Lancet Psychiatry. 2021 Jun;8(6):471-486. doi: 10.1016/S2215-0366(21)00078-X. Lancet Psychiatry. 2021. PMID: 34023019
-
Randomised controlled trials of conventional antipsychotic versus new atypical drugs, and new atypical drugs versus clozapine, in people with schizophrenia responding poorly to, or intolerant of, current drug treatment.Health Technol Assess. 2006 May;10(17):iii-iv, ix-xi, 1-165. doi: 10.3310/hta10170. Health Technol Assess. 2006. PMID: 16707074 Clinical Trial.
References
-
- Higgins, J. P. T. et al. Vol. Available from www.training.cochrane.org/handbook (Cochrane, 2019).
LinkOut - more resources
Full Text Sources