PLK1 phosphorylates RhoGDI1 and promotes cancer cell migration and invasion
- PMID: 38355643
- PMCID: PMC10865702
- DOI: 10.1186/s12935-024-03254-z
PLK1 phosphorylates RhoGDI1 and promotes cancer cell migration and invasion
Abstract
Background: Rho guanine nucleotide dissociation inhibitor 1 (RhoGDI1) plays an important role in diverse cellular processes by regulating Rho guanosine triphosphate (GTP)ases activity. RhoGDI1 phosphorylation regulates the spatiotemporal activation of Rho GTPases during cell migration. In this study, we identified polo-like kinase 1 (PLK1) as a novel kinase of RhoGDI1 and investigated the molecular mechanism by which the interaction between RhoGDI1 and PLK1 regulates cancer cell migration.
Methods: Immunoprecipitation, GST pull-down assay, and proximity ligation assay (PLA) were performed to analyze the interaction between RhoGDI1 and PLK1. In vitro kinase assay and immunoprecipitation were performed with Phospho-(Ser/Thr) antibody. We evaluated RhoA activation using RhoGTPases activity assay. Cell migration and invasion were analyzed by transwell assays.
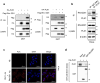
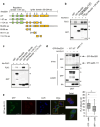
Results: GST pull-down assays and PLA showed that PLK1 directly interacted with RhoGDI1 in vitro and in vivo. Truncation mutagenesis revealed that aa 90-111 of RhoGDI1 are critical for interacting with PLK1. We also showed that PLK1 phosphorylated RhoGDI1 at Thr7 and Thr91, which induces cell motility. Overexpression of the GFP-tagged RhoGDI1 truncated mutant (aa 90-111) inhibited the interaction of PLK1 with RhoGDI1 and attenuated RhoA activation by PLK1. Furthermore, the overexpression of the RhoGDI1 truncated mutant reduced cancer cell migration and invasion in vitro and suppressed lung metastasis in vivo.
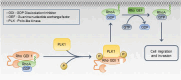
Conclusions: Collectively, we demonstrate that the phosphorylation of RhoGDI1 by PLK1 promotes cancer cell migration and invasion through RhoA activation. This study connects the interaction between PLK1 and RhoGDI1 to the promotion of cancer cell behavior associated with malignant progression, thereby providing opportunities for cancer therapeutic interventions.
Keywords: Cancer; Migration; PLK1; RhoA; RhoGDI1.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no potential conflicts of interest.
Figures




Similar articles
-
NEK2 Phosphorylates RhoGDI1 to Promote Cell Proliferation, Migration and Invasion Through the Activation of RhoA and Rac1 in Colon Cancer Cells.Cells. 2024 Dec 16;13(24):2072. doi: 10.3390/cells13242072. Cells. 2024. PMID: 39768163 Free PMC article.
-
Protein phosphatase 1B dephosphorylates Rho guanine nucleotide dissociation inhibitor 1 and suppresses cancer cell migration and invasion.Cancer Lett. 2018 Mar 28;417:141-151. doi: 10.1016/j.canlet.2018.01.002. Epub 2018 Jan 5. Cancer Lett. 2018. PMID: 29307615
-
EphrinB1 promotes cancer cell migration and invasion through the interaction with RhoGDI1.Oncogene. 2018 Feb 15;37(7):861-872. doi: 10.1038/onc.2017.386. Epub 2017 Oct 23. Oncogene. 2018. PMID: 29059157 Free PMC article.
-
Regulation of Rho GTPases by RhoGDIs in Human Cancers.Cells. 2019 Sep 5;8(9):1037. doi: 10.3390/cells8091037. Cells. 2019. PMID: 31492019 Free PMC article. Review.
-
Cytokinesis and cancer: Polo loves ROCK'n' Rho(A).J Genet Genomics. 2010 Mar;37(3):159-72. doi: 10.1016/S1673-8527(09)60034-5. J Genet Genomics. 2010. PMID: 20347825 Review.
Cited by
-
Synthesis and in vitro evaluation of benzo[b]thiophene-3-carboxylic acid 1,1-dioxide derivatives as anticancer agents targeting the RhoA/ROCK pathway.J Enzyme Inhib Med Chem. 2024 Dec;39(1):2390911. doi: 10.1080/14756366.2024.2390911. Epub 2024 Sep 11. J Enzyme Inhib Med Chem. 2024. PMID: 39258708 Free PMC article.
-
TCF19 expression and significance analysis in breast cancer: integrated bioinformatics analysis and histological validation.Discov Oncol. 2025 Jun 10;16(1):1047. doi: 10.1007/s12672-025-02546-8. Discov Oncol. 2025. PMID: 40493298 Free PMC article.
-
Onvansertib exhibits anti-proliferative and anti-invasive effects in endometrial cancer.Front Pharmacol. 2025 Mar 17;16:1545038. doi: 10.3389/fphar.2025.1545038. eCollection 2025. Front Pharmacol. 2025. PMID: 40166466 Free PMC article.
-
NEK2 Phosphorylates RhoGDI1 to Promote Cell Proliferation, Migration and Invasion Through the Activation of RhoA and Rac1 in Colon Cancer Cells.Cells. 2024 Dec 16;13(24):2072. doi: 10.3390/cells13242072. Cells. 2024. PMID: 39768163 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

