Probiotic performance of B. subtilis MS. 45 improves aquaculture of rainbow trout Oncorhynchus mykiss during acute hypoxia stress
- PMID: 38355704
- PMCID: PMC10866961
- DOI: 10.1038/s41598-024-54380-7
Probiotic performance of B. subtilis MS. 45 improves aquaculture of rainbow trout Oncorhynchus mykiss during acute hypoxia stress
Abstract
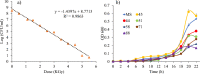
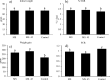
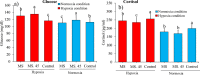
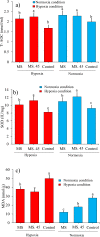
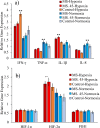
The aim of this study was to produce mutant strains of Bacillus subtilis with high probiotic performance for use in the aquaculture of rainbow trout Oncorhynchus mykiss. The main strain of B. subtilis (MS) was irradiated with gamma rays (5.3 KGy). Subsequently, the B. subtilis mutant strain no. 45 (MS. 45) was selected for bacterial growth performance, resistance to acidic conditions, resistance to bile salts and antibacterial activity against Aeromonas hydrophila and Pseudomonas fluorescens. After 60 days, the rainbow trout (70.25 ± 3.89 g) fed with MS. 45 and MS were exposed to hypoxia stress (dissolved oxygen = 2 ppm). Subsequently, immune indices (lysozyme, bacterial activity and complement activity), hematological indices [hematocrit, hemoglobin, WBC, RBC, mean corpuscular volume (MCV)] and antioxidant factors (T-AOC, SOD and MDA)) were analyzed after and before hypoxia exposure. The expression of immunological genes (IFN-γ, TNF-α, IL-1β, IL-8) in the intestine and the expression of hypoxia-related genes (HIF-1α, HIF-2α, FIH1) in the liver were compared between the different groups under hypoxia and normoxia conditions. Growth, immunological and antioxidant indices improved in group MS. 45 compared to the other groups. Stress indices and associated immunologic and hypoxia expressions under hypoxia and normoxia conditions improved in MS. 45 compared to the other groups. This resulted in improved growth, immunity and stress responses in fish fed with the microbial supplement of MS. 45 (P < 0.05) under hypoxia and normoxia conditions, (P < 0.05), resulting in a significant improvement in trout aquaculture.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Effects of curcumin on haematological values, immunity, antioxidant status and resistance of rainbow trout (Oncorhynchus mykiss) against Aeromonas salmonicida subsp. achromogenes.Fish Shellfish Immunol. 2019 Jun;89:83-90. doi: 10.1016/j.fsi.2019.03.038. Epub 2019 Mar 19. Fish Shellfish Immunol. 2019. PMID: 30898618
-
Effects of dietary cholesterol on antioxidant capacity, non-specific immune response, and resistance to Aeromonas hydrophila in rainbow trout (Oncorhynchus mykiss) fed soybean meal-based diets.Fish Shellfish Immunol. 2013 Jan;34(1):324-31. doi: 10.1016/j.fsi.2012.11.008. Epub 2012 Dec 1. Fish Shellfish Immunol. 2013. PMID: 23207478
-
Protective effects of the prebiotic on the immunological indicators of rainbow trout (Oncorhynchus mykiss) infected with Aeromonas hydrophila.Fish Shellfish Immunol. 2016 Jul;54:589-97. doi: 10.1016/j.fsi.2016.05.010. Epub 2016 May 13. Fish Shellfish Immunol. 2016. PMID: 27184111
-
Mucosal and systemic immune effects of Bacillus subtilis in rainbow trout (Oncorhynchus mykiss).Fish Shellfish Immunol. 2022 May;124:142-155. doi: 10.1016/j.fsi.2022.03.040. Epub 2022 Mar 31. Fish Shellfish Immunol. 2022. PMID: 35367376
-
Modulatory effect of dietary copper nanoparticles and vitamin C supplementations on growth performance, hematological and immune parameters, oxidative status, histology, and disease resistance against Yersinia ruckeri in rainbow trout (Oncorhynchus mykiss).Fish Physiol Biochem. 2022 Feb;48(1):33-51. doi: 10.1007/s10695-021-01036-2. Epub 2021 Nov 30. Fish Physiol Biochem. 2022. PMID: 34850306
Cited by
-
Metabolic Profile of Senegalese Sole (Solea senegalensis) Muscle: Effect of Fish-Macroalgae IMTA-RAS Aquaculture.Molecules. 2025 Jun 9;30(12):2518. doi: 10.3390/molecules30122518. Molecules. 2025. PMID: 40572484 Free PMC article.
References
-
- Merrifield DL, et al. The current status and future focus of probiotic and prebiotic applications for salmonids. Aquaculture. 2010;302:1–18. doi: 10.1016/j.aquaculture.2010.02.007. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

