Autonomous transposons tune their sequences to ensure somatic suppression
- PMID: 38355802
- PMCID: PMC10901741
- DOI: 10.1038/s41586-024-07081-0
Autonomous transposons tune their sequences to ensure somatic suppression
Abstract
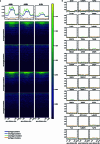
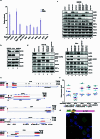
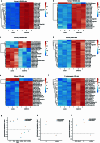
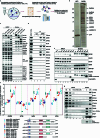
Transposable elements (TEs) are a major constituent of human genes, occupying approximately half of the intronic space. During pre-messenger RNA synthesis, intronic TEs are transcribed along with their host genes but rarely contribute to the final mRNA product because they are spliced out together with the intron and rapidly degraded. Paradoxically, TEs are an abundant source of RNA-processing signals through which they can create new introns1, and also functional2 or non-functional chimeric transcripts3. The rarity of these events implies the existence of a resilient splicing code that is able to suppress TE exonization without compromising host pre-mRNA processing. Here we show that SAFB proteins protect genome integrity by preventing retrotransposition of L1 elements while maintaining splicing integrity, via prevention of the exonization of previously integrated TEs. This unique dual role is possible because of L1's conserved adenosine-rich coding sequences that are bound by SAFB proteins. The suppressive activity of SAFB extends to tissue-specific, giant protein-coding cassette exons, nested genes and Tigger DNA transposons. Moreover, SAFB also suppresses LTR/ERV elements in species in which they are still active, such as mice and flies. A significant subset of splicing events suppressed by SAFB in somatic cells are activated in the testis, coinciding with low SAFB expression in postmeiotic spermatids. Reminiscent of the division of labour between innate and adaptive immune systems that fight external pathogens, our results uncover SAFB proteins as an RNA-based, pattern-guided, non-adaptive defence system against TEs in the soma, complementing the RNA-based, adaptive Piwi-interacting RNA pathway of the germline.
© 2024. The Author(s).
Conflict of interest statement
I.A.I. and T.A. are inventors on a patent application (no. EP3325621B1, European Patent Office) regarding the s-oligo design used in FLASH experiments. Z.D.S. is a cofounder and scientific advisor of Harbinger Health.
Figures










References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

