Visuo-frontal interactions during social learning in freely moving macaques
- PMID: 38355804
- PMCID: PMC10959748
- DOI: 10.1038/s41586-024-07084-x
Visuo-frontal interactions during social learning in freely moving macaques
Erratum in
-
Publisher Correction: Visuo-frontal interactions during social learning in freely moving macaques.Nature. 2024 Apr;628(8008):E3. doi: 10.1038/s41586-024-07301-7. Nature. 2024. PMID: 38519583 Free PMC article. No abstract available.
Abstract
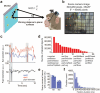
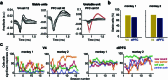
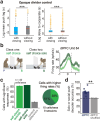
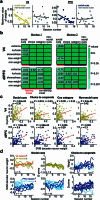
Social interactions represent a ubiquitous aspect of our everyday life that we acquire by interpreting and responding to visual cues from conspecifics1. However, despite the general acceptance of this view, how visual information is used to guide the decision to cooperate is unknown. Here, we wirelessly recorded the spiking activity of populations of neurons in the visual and prefrontal cortex in conjunction with wireless recordings of oculomotor events while freely moving macaques engaged in social cooperation. As animals learned to cooperate, visual and executive areas refined the representation of social variables, such as the conspecific or reward, by distributing socially relevant information among neurons in each area. Decoding population activity showed that viewing social cues influences the decision to cooperate. Learning social events increased coordinated spiking between visual and prefrontal cortical neurons, which was associated with improved accuracy of neural populations to encode social cues and the decision to cooperate. These results indicate that the visual-frontal cortical network prioritizes relevant sensory information to facilitate learning social interactions while freely moving macaques interact in a naturalistic environment.
© 2024. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
The authors declare no competing interests.
Figures











References
-
- Emery, N. J. The eyes have it: the neuroethology, function and evolution of social gaze. Neurosci. Biobehav. Rev.24, 581–604 (2000). - PubMed
-
- Nahm, F. K., Perret, A., Amaral, D. G. & Albright, T. D. How do monkeys look at faces? J. Cogn. Neurosci.9, 611–623 (1997). - PubMed
-
- Emery, N. J., Lorincz, E. N., Perrett, D. I., Oram, M. W. & Baker, C. I. Gaze following and joint attention in rhesus monkeys (Macaca mulatta). J. Comp. Psychol.111, 286–293 (1997). - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

