Standardization of the protocol for oral cavity examination and collecting of the biological samples for microbiome research using the next-generation sequencing (NGS): own experience with the COVID-19 patients
- PMID: 38355866
- PMCID: PMC10867075
- DOI: 10.1038/s41598-024-53992-3
Standardization of the protocol for oral cavity examination and collecting of the biological samples for microbiome research using the next-generation sequencing (NGS): own experience with the COVID-19 patients
Abstract
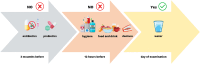
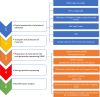
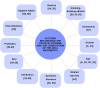
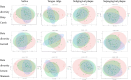
To date, publications have shown that compositions of oral microbiota differ depending on their habitats (e.g. tongue, tonsils, pharynx). The absence of set standards for the choice of the areas and conditions of material collection makes the oral microbiome one of the most difficult environments for a comparative analysis with other researchers, which is a meaningful limitation during an assessment of the potential effects of microorganisms as biomarkers in the courses of various human diseases. Therefore, standardisation of basic conditions of a dental examination and collection of material for the next generation sequencing (NGS) is worth attempting. The standardisation of the dental exam and collection of the clinical materials: saliva, swab from the tongue ridge, hard palate, palatine tonsils and oropharynx, supragingival plaque and subgingival plaque. Protocol involved the patients (n = 60), assigned to 3 groups: I-COVID-19 convalescents who received antibiotics, n = 17, II-COVID-19 convalescents, n = 23 and III-healthy individuals, n = 20. The collected biological samples were used to conduct NGS (16S rRNA). The conditions of patient preparation for collecting biological materials as well as the schedule of dental examination, were proposed. Based on the research conducted, we have indicated the dental indicators that best differentiate the group of COVID-19 patients (groups I and II) from healthy people (group III). These include the DMFT, D and BOP indices. The use of alpha and beta diversity analysis provided an overall insight into the diversity of microbial communities between specific niches and patient groups. The most different diversity between the studied group of patients (group II) and healthy people (group III) was noted in relation to the supragingival plaque. The order of activities during the dental exam as well as while collecting and securing clinical materials is particularly important to avoid technical errors and material contamination which may result in erroneous conclusions from the analyses of the results of sensitive tests such as the NGS. It has been shown that the dental indices: DMFT, D number, PI and BOP are the best prognostic parameters to assess the oral health. Based on beta diversity the most sensitive niche and susceptible to changes in the composition of the microbiota is the supragingival plaque. The procedures developed by our team can be applied as ready-to-use forms in studies conducted by other researchers.
Keywords: Calibration; Dentistry; Microbiota; Next generation sequencing; Oral health; Oral hygiene; Standardization.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Oral microbiota study of the patients after hospitalisation for COVID-19, considering selected dental indices and antibiotic therapy using the next generation sequencing method (NGS).J Oral Microbiol. 2023 Oct 11;15(1):2264591. doi: 10.1080/20002297.2023.2264591. eCollection 2023. J Oral Microbiol. 2023. PMID: 37840855 Free PMC article.
-
Defining the oral microbiome by whole-genome sequencing and resistome analysis: the complexity of the healthy picture.BMC Microbiol. 2020 May 18;20(1):120. doi: 10.1186/s12866-020-01801-y. BMC Microbiol. 2020. PMID: 32423437 Free PMC article.
-
VMG II transport medium stabilises oral microbiome samples for Next-Generation Sequencing.J Microbiol Methods. 2018 Jan;144:91-98. doi: 10.1016/j.mimet.2017.11.012. Epub 2017 Nov 22. J Microbiol Methods. 2018. PMID: 29155022
-
Next Generation Sequencing and the Microbiome of Chronic Rhinosinusitis: A Primer for Clinicians and Review of Current Research, Its Limitations, and Future Directions.Ann Otol Rhinol Laryngol. 2016 Aug;125(8):613-21. doi: 10.1177/0003489416641429. Epub 2016 Apr 7. Ann Otol Rhinol Laryngol. 2016. PMID: 27056556 Review.
-
Understanding and overcoming the pitfalls and biases of next-generation sequencing (NGS) methods for use in the routine clinical microbiological diagnostic laboratory.Eur J Clin Microbiol Infect Dis. 2019 Jun;38(6):1059-1070. doi: 10.1007/s10096-019-03520-3. Epub 2019 Mar 5. Eur J Clin Microbiol Infect Dis. 2019. PMID: 30834996 Free PMC article. Review.
Cited by
-
Engineered Tissue Models to Decode Host-Microbiota Interactions.Adv Sci (Weinh). 2025 Jun;12(23):e2417687. doi: 10.1002/advs.202417687. Epub 2025 May 14. Adv Sci (Weinh). 2025. PMID: 40364768 Free PMC article. Review.
-
Mechanisms of crosstalk between the oropharyngeal microbiome and human papillomavirus in oropharyngeal carcinogenesis: a mini review.Front Oncol. 2024 Aug 15;14:1425545. doi: 10.3389/fonc.2024.1425545. eCollection 2024. Front Oncol. 2024. PMID: 39211550 Free PMC article. Review.
-
Variations in oral health outcomes and mycobiome composition among COVID-19 convalescents.Sci Rep. 2025 Jul 1;15(1):21638. doi: 10.1038/s41598-025-05078-x. Sci Rep. 2025. PMID: 40596128 Free PMC article.
-
The Role of Microbiota in Upper Gastrointestinal Cancers.Cancers (Basel). 2025 May 21;17(10):1719. doi: 10.3390/cancers17101719. Cancers (Basel). 2025. PMID: 40427216 Free PMC article. Review.
-
Effects of Mint Oils on the Human Oral Microbiome: A Pilot Study.Microorganisms. 2024 Jul 27;12(8):1538. doi: 10.3390/microorganisms12081538. Microorganisms. 2024. PMID: 39203382 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

