Dual anti-HER2/EGFR inhibition synergistically increases therapeutic effects and alters tumor oxygenation in HNSCC
- PMID: 38355949
- PMCID: PMC10866896
- DOI: 10.1038/s41598-024-52897-5
Dual anti-HER2/EGFR inhibition synergistically increases therapeutic effects and alters tumor oxygenation in HNSCC
Abstract
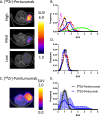
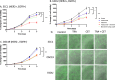
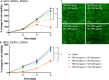
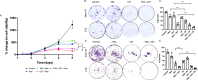
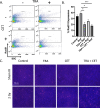
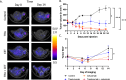
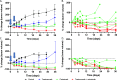
Epidermal growth factor receptor (EGFR), human epidermal growth factor receptor 2 (HER2), and hypoxia are associated with radioresistance. The goal of this study is to study the synergy of anti-HER2, trastuzumab, and anti-EGFR, cetuximab, and characterize the tumor microenvironment components that may lead to increased radiation sensitivity with dual anti-HER2/EGFR therapy in head and neck squamous cell carcinoma (HNSCC). Positron emission tomography (PET) imaging ([89Zr]-panitumumab and [89Zr]-pertuzumab) was used to characterize EGFR and HER2 in HNSCC cell line tumors. HNSCC cells were treated with trastuzumab, cetuximab, or combination followed by radiation to assess for viability and radiosensitivity (colony forming assay, immunofluorescence, and flow cytometry). In vivo, [18F]-FMISO-PET imaging was used to quantify changes in oxygenation during treatment. Bliss Test of Synergy was used to identify combination treatment synergy. Quantifying EGFR and HER2 receptor expression revealed a 50% increase in heterogeneity of HER2 relative to EGFR. In vitro, dual trastuzumab-cetuximab therapy shows significant decreases in DNA damage response and increased response to radiation therapy (p < 0.05). In vivo, tumors treated with dual anti-HER2/EGFR demonstrated decreased tumor hypoxia, when compared to single agent therapies. Dual trastuzumab-cetuximab demonstrates synergy and can affect tumor oxygenation in HNSCC. Combination trastuzumab-cetuximab modulates the tumor microenvironment through reductions in tumor hypoxia and induces sustained treatment synergy.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

