An inflamed tumor cell subpopulation promotes chemotherapy resistance in triple negative breast cancer
- PMID: 38355954
- PMCID: PMC10866903
- DOI: 10.1038/s41598-024-53999-w
An inflamed tumor cell subpopulation promotes chemotherapy resistance in triple negative breast cancer
Abstract
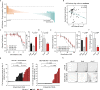
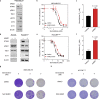
Individual cancers are composed of heterogeneous tumor cells with distinct phenotypes and genotypes, with triple negative breast cancers (TNBC) demonstrating the most heterogeneity among breast cancer types. Variability in transcriptional phenotypes could meaningfully limit the efficacy of monotherapies and fuel drug resistance, although to an unknown extent. To determine if transcriptional differences between tumor cells lead to differential drug responses we performed single cell RNA-seq on cell line and PDX models of breast cancer revealing cell subpopulations in states associated with resistance to standard-of-care therapies. We found that TNBC models contained a subpopulation in an inflamed cellular state, often also present in human breast cancer samples. Inflamed cells display evidence of heightened cGAS/STING signaling which we demonstrate is sufficient to cause tumor cell resistance to chemotherapy. Accordingly, inflamed cells were enriched in human tumors taken after neoadjuvant chemotherapy and associated with early recurrence, highlighting the potential for diverse tumor cell states to promote drug resistance.
© 2024. The Author(s).
Conflict of interest statement
S.B. consults with and/or receives research funding from Pfizer, Ideaya Biosciences and Revolution Medicines and is an employee and shareholder in Rezo Therapeutics. No other authors have any conflict of interest on this work.
Figures




References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

