Long-term and real-world safety and efficacy of retroviral gene therapy for adenosine deaminase deficiency
- PMID: 38355973
- PMCID: PMC7615698
- DOI: 10.1038/s41591-023-02789-4
Long-term and real-world safety and efficacy of retroviral gene therapy for adenosine deaminase deficiency
Abstract
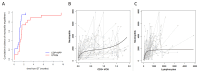
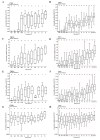
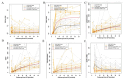
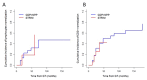
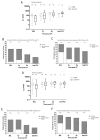
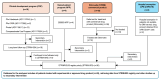
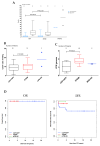
Adenosine deaminase (ADA) deficiency leads to severe combined immunodeficiency (SCID). Previous clinical trials showed that autologous CD34+ cell gene therapy (GT) following busulfan reduced-intensity conditioning is a promising therapeutic approach for ADA-SCID, but long-term data are warranted. Here we report an analysis on long-term safety and efficacy data of 43 patients with ADA-SCID who received retroviral ex vivo bone marrow-derived hematopoietic stem cell GT. Twenty-two individuals (median follow-up 15.4 years) were treated in the context of clinical development or named patient program. Nineteen patients were treated post-marketing authorization (median follow-up 3.2 years), and two additional patients received mobilized peripheral blood CD34+ cell GT. At data cutoff, all 43 patients were alive, with a median follow-up of 5.0 years (interquartile range 2.4-15.4) and 2 years intervention-free survival (no need for long-term enzyme replacement therapy or allogeneic hematopoietic stem cell transplantation) of 88% (95% confidence interval 78.7-98.4%). Most adverse events/reactions were related to disease background, busulfan conditioning or immune reconstitution; the safety profile of the real world experience was in line with premarketing cohort. One patient from the named patient program developed a T cell leukemia related to treatment 4.7 years after GT and is currently in remission. Long-term persistence of multilineage gene-corrected cells, metabolic detoxification, immune reconstitution and decreased infection rates were observed. Estimated mixed-effects models showed that higher dose of CD34+ cells infused and younger age at GT affected positively the plateau of CD3+ transduced cells, lymphocytes and CD4+ CD45RA+ naive T cells, whereas the cell dose positively influenced the final plateau of CD15+ transduced cells. These long-term data suggest that the risk-benefit of GT in ADA remains favorable and warrant for continuing long-term safety monitoring. Clinical trial registration: NCT00598481 , NCT03478670 .
© 2024. The Author(s), under exclusive licence to Springer Nature America, Inc.
Conflict of interest statement
The San Raffaele Telethon Institute for Gene Therapy (SR-Tiget) is a joint venture between the Telethon Foundation and Ospedale San Raffaele (OSR). Gene therapy for ADA-SCID was developed at SR-Tiget and licensed to GlaxoSmithKline (GSK) in 2010. The treatments under NPP and Hospital Exemption were provided free of charge by GSK. Strimvelis Marketing Authorization in Europe occurred in 2016 (under GSK holding) and then transferred to Orchard Therapeutics (Netherlands) B.V. in 2018, which divested the program and transfer the authorization to Fondazione Telethon that became the holder in July 2023. The product, apart EU, is also currently approved in Iceland, Norway, Liechtenstein, and UK. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript. The authors received no specific funding for this work.
A. Aiuti receives funding from Fondazione Telethon for other research projects.
A. Aiuti was the PI of pilot and pivotal and long-term F-U study. SR-Tiget clinical trial of gene therapy for ADA SCID. M.P. Cicalese and M. Migliavacca are PI and deputy PI, respectively of the Strimvelis Registry, RIS and RMMs studies. All authors declare no other competing interests.
Figures




References
-
- Hassan A, et al. Inborn Errors Working Party of the European Group for Blood and Marrow Transplantation and European Society for Immunodeficiency. Outcome of hematopoietic stem cell transplantation for adenosine deaminase-deficient severe combined immunodeficiency. Blood. 2012;120:3615–3624. - PubMed
-
- Lankester A, et al. Hematopoietic cell transplantation in severe combined immunodeficiency: The SCETIDE 2006-2014 European cohort. J Allergy Clin Immunol. 2022;149:1744–1754. - PubMed
MeSH terms
Substances
Supplementary concepts
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

