Association of region-specific hippocampal reduction of neurogranin with inflammasome proteins in post mortem brains of Alzheimer's disease
- PMID: 38356472
- PMCID: PMC10865487
- DOI: 10.1002/trc2.12444
Association of region-specific hippocampal reduction of neurogranin with inflammasome proteins in post mortem brains of Alzheimer's disease
Abstract
Introduction: Neurogranin (Ng) is considered a biomarker for synaptic dysfunction in Alzheimer's disease (AD). In contrast, the inflammasome complex has been shown to exacerbate AD pathology.
Methods: We investigated the protein expression, morphological differences of Ng, and correlated Ng to hyperphosphorylated tau in the post mortem brains of 17 AD cases and 17 age- and sex-matched controls. In addition, we correlated the Ng expression with two different epitopes of apoptosis-associated speck-like protein containing a caspase recruitment domain (ASC).
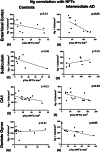
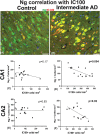
Results: We show a reduction of Ng immunopositive neurons and morphological differences in AD compared to controls. Ng immunostaining was negatively correlated with neurofibrillary tangles, humanized anti-ASC (IC100) positive neurons and anti-ASC positive microglia, in AD.
Discussion: The finding of a negative correlation between Ng and ASC speck protein expression in post mortem brains of AD suggests that the activation of inflammasome/ASC speck pathway may play an important role in synaptic degeneration in AD.
Highlights: We show the role that neurogranin plays on post-synaptic signaling in specific hippocampal regions.We demonstrate that there could be clinical implications of using neurogranin as a biomarker for dementia.We describe the loss of plasticity and neuronal scaffolding proteins in the present of AD pathology.We show the response of neuroinflammation when tau proteins phosphorylate in hippocampal neurons.We show that there is a potential therapeutic target for the inflammasome, and future studies may show that IC100, a humanized monoclonal antibody directed against ASC, may slow the progression of neurodegeneration.
Keywords: Alzheimer's disease; axon; inflammasome; microglia; neurogranin.
© 2024 The Authors. Alzheimer's & Dementia: Translational Research & Clinical Interventions published by Wiley Periodicals LLC on behalf of Alzheimer's Association.
Conflict of interest statement
KB and HZ have served on scientific advisory boards and/or as consultants for Abbvie, Acumen, Alector, Alzinova, ALZPath, Annexon, Apellis, Artery Therapeutics, AZTherapies, Cognito Therapeutics, CogRx, Denali, Eisai, Nervgen, Novo Nordisk, Optoceutics, Passage Bio, Pinteon Therapeutics, Prothena, Red Abbey Labs, reMYND, Roche, Samumed, Siemens Healthineers, Triplet Therapeutics, and Wave, have given lectures in symposia sponsored by Cellectricon, Fujirebio, Alzecure, Biogen, and Roche, and are co‐founders of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program (outside submitted work).JPdRV, HMB, RWK, and WDD are co‐founders and managing members of InflamaCORE, LLC and have licensed patents on inflammasome proteins as biomarkers of injury and disease as well as on targeting inflammasome proteins for therapeutic purposes. JPdRV, HMB, RWK, and WDD are Scientific Advisory Board Members of ZyVersa Therapeutics. The other authors declare no conflicts of interest. Author disclosures are available in the supporting information.
Figures



Similar articles
-
Identification of inflammasome signaling proteins in neurons and microglia in early and intermediate stages of Alzheimer's disease.Brain Pathol. 2023 Jul;33(4):e13142. doi: 10.1111/bpa.13142. Epub 2022 Dec 29. Brain Pathol. 2023. PMID: 36579934 Free PMC article.
-
Genetic predisposition to Alzheimer's disease alters inflammasome activity after traumatic brain injury.Transl Res. 2023 Jul;257:66-77. doi: 10.1016/j.trsl.2023.02.001. Epub 2023 Feb 8. Transl Res. 2023. PMID: 36758791 Free PMC article.
-
Ethanol consumption aggravates amyloid pathology and neuroinflammation in Alzheimer's disease associated with inflammasome activation and ASC speck propagation.J Neuroinflammation. 2025 Jul 15;22(1):183. doi: 10.1186/s12974-025-03501-8. J Neuroinflammation. 2025. PMID: 40665351 Free PMC article.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
Plasma and cerebrospinal fluid amyloid beta for the diagnosis of Alzheimer's disease dementia and other dementias in people with mild cognitive impairment (MCI).Cochrane Database Syst Rev. 2014 Jun 10;2014(6):CD008782. doi: 10.1002/14651858.CD008782.pub4. Cochrane Database Syst Rev. 2014. PMID: 24913723 Free PMC article.
Cited by
-
Post-stroke hippocampal neurogenesis is impaired by microvascular dysfunction and PI3K signaling in cerebral amyloid angiopathy.Cell Rep. 2024 Oct 22;43(10):114848. doi: 10.1016/j.celrep.2024.114848. Epub 2024 Oct 10. Cell Rep. 2024. PMID: 39392753 Free PMC article.
-
Effects of Space Flight on Inflammasome Activation in the Brain of Mice.Cells. 2025 Mar 12;14(6):417. doi: 10.3390/cells14060417. Cells. 2025. PMID: 40136666 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
