Phase 1 study of latozinemab in progranulin-associated frontotemporal dementia
- PMID: 38356474
- PMCID: PMC10865485
- DOI: 10.1002/trc2.12452
Phase 1 study of latozinemab in progranulin-associated frontotemporal dementia
Abstract
Introduction: Heterozygous mutations in the GRN gene lead to reduced progranulin (PGRN) levels in plasma and cerebrospinal fluid (CSF) and are causative of frontotemporal dementia (FTD) with > 90% penetrance. Latozinemab is a human monoclonal immunoglobulin G1 antibody that is being developed to increase PGRN levels in individuals with FTD caused by heterozygous loss-of-function GRN mutations.
Methods: A first-in-human phase 1 study was conducted to evaluate the safety, tolerability, pharmacokinetics (PK), and pharmacodynamics (PD) of multiple-dose intravenous administration of latozinemab in eight symptomatic participants with FTD caused by a heterozygous loss-of-function GRN mutation (FTD-GRN).
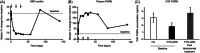
Results: Latozinemab demonstrated favorable safety and PK/PD profiles. Multiple-dose administration of latozinemab increased plasma and CSF PGRN levels in participants with FTD-GRN to levels that approximated those seen in healthy volunteers.
Discussion: Data from the first-in-human phase 1 study support further development of latozinemab for the treatment of FTD-GRN.
Highlights: GRN mutations decrease progranulin (PGRN) and cause frontotemporal dementia (FTD).Latozinemab is being developed as a PGRN-elevating therapy.Latozinemab demonstrated a favorable safety profile in a phase 1 clinical trial.Latozinemab increased PGRN levels in the CNS of symptomatic FTD-GRN participants.
Keywords: disease‐modifying therapy; frontotemporal dementia; latozinemab; loss‐of‐function GRN mutation; phase 1 clinical trial; progranulin; sortilin.
© 2024 Alector. Alzheimer's & Dementia: Translational Research & Clinical Interventions published by Wiley Periodicals LLC on behalf of Alzheimer's Association.
Conflict of interest statement
Michael Ward was an employee of Alector at the time of the study; has received consulting fees and travel support from Alector; is listed on patents with Alector; and has stock and stock options with Alector. Lawrence P. Carter is an employee of Alector; has received travel support from Alector; has stock and stock options with Alector; and has received honoraria as an invited speaker from the University of Michigan. Julie Y. Huang is an employee of Alector; has received travel support from Alector; is listed on provisional patents for Alector; and has received stock and stock options from Alector. Daniel Maslyar was an employee of Alector at the time of the study; has received consulting fees and travel support to conferences from Alector; and is an Alector stockholder. Balasubrahmanyam Budda is an employee of Alector; has received support for travel to conferences from Alector; and has received stock and stock options from Alector. Robert Paul was an employee of Alector at the time of the study; is listed on patents with Alector; and has received stock and stock options from Alector. Arnon Rosenthal is an employee of Alector; is listed on multiple patents for progranulin elevating drugs as a co‐inventor; and has received stock and stock options from Alector. Author disclosures are available in the supporting information.
Figures


References
-
- Ber ILe, Camuzat A, Hannequin D, et al. Phenotype variability in progranulin mutation carriers: a clinical, neuropsychological, imaging and genetic study. Brain. 2008;131:732‐746. - PubMed
-
- Seelaar H, Rohrer JD, Pijnenburg YA, Fox NC, van Swieten JC. Clinical, genetic and pathological heterogeneity of frontotemporal dementia: a review. J Neurol Neurosurg Psychiatry. 2011;82:476‐486. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
