Case report: Identification of a novel variant p.Gly215Arg in the CHN1 gene causing Moebius syndrome
- PMID: 38356699
- PMCID: PMC10865368
- DOI: 10.3389/fgene.2024.1291063
Case report: Identification of a novel variant p.Gly215Arg in the CHN1 gene causing Moebius syndrome
Abstract
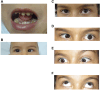
Background: Moebius Syndrome (MBS) is a rare congenital neurological disorder characterized by paralysis of facial nerves, impairment of ocular abduction and other variable abnormalities. MBS has been attributed to both environmental and genetic factors as potential causes. Until now only two genes, PLXND1 and REV3L have been identified to cause MBS. Results: We present a 9-year-old male clinically diagnosed with MBS, presenting facial palsy, altered ocular mobility, microglossia, dental anomalies and congenital torticollis. Radiologically, he lacks both abducens nerves and shows altered symmetry of both facial and vestibulocochlear nerves. Whole-exome sequence identified a de novo missense variant c.643G>A; p.Gly215Arg in CHN1, encoding the α2-chimaerin protein. The p.Gly215Arg variant is located in the C1 domain of CHN1 where other pathogenic gain of function variants have been reported. Bioinformatic analysis and molecular structural modelling predict a deleterious effect of the missense variant on the protein function. Conclusion: Our findings support that pathogenic variants in the CHN1 gene may be responsible for different cranial congenital dysinnervation syndromes, including Moebius and Duane retraction syndromes. We propose to include CHN1 in the genetic diagnoses of MBS.
Keywords: CHN1; congenital dysinnervation syndromes; genetic diagnosis; moebius syndrome; novel variant.
Copyright © 2024 Manso-Bazús, Spataro, Gabau, Beltrán-Salazar, Trujillo-Quintero, Capdevila, Brunet-Vega, Baena, Jeyaprakash, Martinez-Glez and Ruiz.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures



References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

