Association between Atopic Dermatitis and Colorectal Cancer: TET2 as a Shared Gene Signature and Prognostic Biomarker
- PMID: 38356721
- PMCID: PMC10861813
- DOI: 10.7150/jca.92238
Association between Atopic Dermatitis and Colorectal Cancer: TET2 as a Shared Gene Signature and Prognostic Biomarker
Abstract
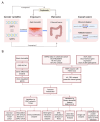
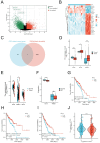
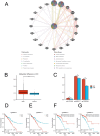
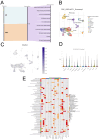
Background: Recent studies have linked atopic dermatitis (AD) to colorectal cancer (CRC) risk. Their causality and potential molecular mechanisms remain unclear. Methods: We performed Mendelian randomization (MR) analysis to evaluate the causality between AD and CRC. Summary statistic data-based Mendelian randomization (SMR) analysis was used to identify CRC-related causal genes. Transcriptome analyses and immunohistochemical methods were applied to investigate the shared gene signature and potential mechanisms that contribute to the pathogenesis of both AD and CRC. A predictive analysis was performed to examine the shared gene signature associated with immunotherapy response in CRC. Results: MR analysis indicated a causal association between AD and a decreased risk of CRC. SMR analysis uncovered TET2 as a CRC-related causal gene, showing an inverse relationship with the risk of CRC. Transcriptome analyses identified TET2 as a shared gene signature between AD and CRC. Decreased TET2 expression is associated with impaired demethylation and worse prognosis in CRC patients. We observed ten pathways related to the inflammatory response and immune regulation that may be shared mechanisms underlying both AD and CRC. These findings were validated through single-cell analysis. TET2 shows promise as a powerful predictive biomarker for cancer prognosis and immunotherapy response in CRC. Conclusion: There is a causal association between AD and a decreased risk of CRC. AD may influence the occurrence of CRC by modulating immune and inflammatory responses. TET2 could serve as a potential biomarker for prognosis and may be considered a novel therapeutic target for methylation and immune-related interventions.
Keywords: Mendelian randomization; TET2; WGCNA.; atopic dermatitis; colorectal cancer.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures


Similar articles
-
Integrated multi-omics analyses revealed the association between rheumatoid arthritis and colorectal cancer: MYO9A as a shared gene signature and an immune-related therapeutic target.BMC Cancer. 2024 Jun 10;24(1):714. doi: 10.1186/s12885-024-12466-5. BMC Cancer. 2024. PMID: 38858644 Free PMC article.
-
Mendelian randomization and transcriptomic analysis reveal a positive cause-and-effect relationship between Alzheimer's disease and colorectal cancer.Transl Oncol. 2025 Jan;51:102169. doi: 10.1016/j.tranon.2024.102169. Epub 2024 Nov 27. Transl Oncol. 2025. PMID: 39608211 Free PMC article.
-
Mendelian randomization indicates that atopic dermatitis contributes to the occurrence of diabetes.BMC Med Genomics. 2023 Jun 15;16(1):132. doi: 10.1186/s12920-023-01575-y. BMC Med Genomics. 2023. PMID: 37322504 Free PMC article.
-
Mendelian Randomization Combined with Single-Cell Transcriptome Analysis Reveals the Role of the Key Gene PCLAF in the Pathogenesis of Atopic Dermatitis.Clin Cosmet Investig Dermatol. 2025 Apr 8;18:867-882. doi: 10.2147/CCID.S506139. eCollection 2025. Clin Cosmet Investig Dermatol. 2025. PMID: 40225314 Free PMC article.
-
Association Between Atopic Dermatitis and Major Cardiovascular Outcomes: a Two-Sample Mendelian Randomization Study.Dermatol Pract Concept. 2022 Oct 1;12(4):e2022165. doi: 10.5826/dpc.1204a165. eCollection 2022 Nov. Dermatol Pract Concept. 2022. PMID: 36534570 Free PMC article. Review.
Cited by
-
Multidimensional Assessment of Psychiatric Adverse Events Related to Proton Pump Inhibitors: A Real-World, Pharmacovigilance Study.CNS Neurosci Ther. 2025 May;31(5):e70436. doi: 10.1111/cns.70436. CNS Neurosci Ther. 2025. PMID: 40365732 Free PMC article.
-
Association Between Biologics and Janus Kinase Inhibitors With Inflammatory Bowel Disease as Paradoxical Reactions: A Real-World Assessment.United European Gastroenterol J. 2025 May;13(4):531-541. doi: 10.1002/ueg2.12719. Epub 2024 Dec 16. United European Gastroenterol J. 2025. PMID: 39676727 Free PMC article.
-
Inflammasomes Are Influenced by Epigenetic and Autophagy Mechanisms in Colorectal Cancer Signaling.Int J Mol Sci. 2024 Jun 3;25(11):6167. doi: 10.3390/ijms25116167. Int J Mol Sci. 2024. PMID: 38892354 Free PMC article. Review.
References
-
- Turner MC, Chen Y, Krewski D, Ghadirian P. An overview of the association between allergy and cancer. Int J Cancer. 2006;118:3124–32. - PubMed
-
- Vojtechova P, Martin RM. The association of atopic diseases with breast, prostate, and colorectal cancers: a meta-analysis. Cancer Causes Control. 2009;20:1091–105. - PubMed
-
- Laughter MR, Maymone MBC, Mashayekhi S, Arents BWM, Karimkhani C, Langan SM. et al. The global burden of atopic dermatitis: lessons from the Global Burden of Disease Study 1990-2017. Br J Dermatol. 2021;184:304–9. - PubMed
-
- Arana A, Wentworth CE, Fernández-Vidaurre C, Schlienger RG, Conde E, Arellano FM. Incidence of cancer in the general population and in patients with or without atopic dermatitis in the U.K. Br J Dermatol. 2010;163:1036–43. - PubMed
-
- Hagströmer L, Ye W, Nyrén O, Emtestam L. Incidence of cancer among patients with atopic dermatitis. Arch Dermatol. 2005;141:1123–7. - PubMed
LinkOut - more resources
Full Text Sources

