Efficacy and safety of stapokibart (CM310) in uncontrolled seasonal allergic rhinitis (MERAK): an investigator-initiated, placebo-controlled, randomised, double-blind, phase 2 trial
- PMID: 38356731
- PMCID: PMC10864214
- DOI: 10.1016/j.eclinm.2024.102467
Efficacy and safety of stapokibart (CM310) in uncontrolled seasonal allergic rhinitis (MERAK): an investigator-initiated, placebo-controlled, randomised, double-blind, phase 2 trial
Abstract
Background: There is no trial to assess the benefits of periodically using biologics during the pollen season in patients with uncontrolled seasonal allergic rhinitis (SAR), who have moderate-to-severe symptoms even after standard-of-care. This trial aimed to evaluate the efficacy and safety of the add-on administration of stapokibart, a humanised monoclonal antibody that targets interleukin-4 receptor alpha, in patients with uncontrolled SAR.
Methods: In this investigator-initiated, randomised, double-blind, placebo-controlled trial, eligible patients received either stapokibart 600-300 mg weekly (QW), every 2 weeks (Q2W), or placebo QW for 4 weeks. All patients were given mometasone furoate nasal spray and loratadine throughout the trial. The primary endpoint was the mean change from baseline in daily reflective total nasal symptom score (rTNSS) during 2-week treatment. Secondary efficacy outcomes included: the mean change from baseline in daily rTNSS during 4-week treatment; the mean changes and the mean percentage changes from baseline during 2-week and 4-week treatment in 1) daily rTNSS and reflective total ocular symptom score (rTOSS), 2) morning (AM)/evening (PM) rTNSS and rTOSS, 3) AM instantaneous total nasal symptom score (iTNSS) and instantaneous total ocular symptom score (iTOSS), 4) individual nasal and ocular symptoms; the change from baseline in Rhinoconjunctivitis Quality of-Life Questionnaire score during 4-week treatment. Exploratory endpoints included the change of prespecified markers related to type 2 inflammation pre- and post-treatment. Safety, immunogenicity, and pharmacokinetics were also evaluated. This study is registered with www.clinicaltrials.gov (NCT05470647).
Findings: Between August 17, 2022, and December 28, 2022, 92 patients with uncontrolled SAR were enrolled from 4 centres in China and randomly assigned to receive stapokibart 600-300 mg QW (n = 31), stapokibart 600-300 mg Q2W (n = 30), or placebo QW (n = 31), of whom 86 (93%) completed the study. Both stapokibart Q2W and QW did not significantly improve mean change from baseline in daily rTNSS compared with placebo in 2 weeks. The least-squares (LS) mean differences (97.5% confidence interval [CI]) compared with placebo were -1.0 (-2.3, 0.2) in stapokibart Q2W group (p = 0.065) and -0.2 (-1.5, 1.0) in stapokibart QW group (p = 0.67). For the secondary outcomes, compared with placebo, stapokibart Q2W presented significant improvements in the mean percentage change from baseline in daily rTNSS in 2 weeks (LS mean difference -12.9%, 95% CI -25.3%, -0.4%, p = 0.043), as well as AM iTNSS over 2 weeks (LS mean difference -17.4%, 95% CI -31.0%, -3.8%, p = 0.013) and 4 weeks (LS mean difference -15.4%, 95% CI -29.0%, -1.9%, p = 0.026). Additionally, the nasal congestion score was significantly lower in stapokibart Q2W than placebo during 2-week (LS mean difference -0.4, 95% CI -0.7, -0.1, p = 0.014) and 4-week (LS mean difference -0.4, 95% CI -0.7, -0.04, p = 0.028) treatment. Treatment-emergent adverse events (TEAEs) occurred in 48% (15/31), 33% (10/30), and 61% (19/31) of patients receiving stapokibart QW, Q2W, and placebo, respectively. Most reported TEAEs were sinus bradycardia, hyperlipidaemia, and blood uric acid increased.
Interpretation: In this phase 2 trial, both stapokibart regimens had an acceptable safety and tolerability profile but did not significantly improve daily rTNSS in patients with uncontrolled SAR. The efficacy of stapokibart in patients with uncontrolled SAR is being further investigated in ongoing phase 3 trials (clinicaltrials.gov, NCT05908032).
Funding: Ministry of Science and Technology of the People's Republic of China; Ministry of Education of the People's Republic of China; National Natural Science Foundation of China; Chinese Academy of Medical Sciences.
Keywords: Co-seasonal application; Interleukin-4 receptor alpha; Monoclonal antibody; Seasonal allergic rhinitis; Uncontrolled.
© 2024 The Author(s).
Conflict of interest statement
LZ was supported by grants from the Ministry of Science and Technology of the People's Republic of China; the Ministry of Education of the People's Republic of China and the Chinese Academy of Medical Sciences; CW declares support from the National Natural Science Foundation of China.
Figures


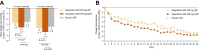

Similar articles
-
Stapokibart for moderate-to-severe seasonal allergic rhinitis: a randomized phase 3 trial.Nat Med. 2025 Jul;31(7):2213-2221. doi: 10.1038/s41591-025-03651-5. Epub 2025 Apr 4. Nat Med. 2025. PMID: 40186079 Free PMC article. Clinical Trial.
-
Stapokibart (CM310) in patients with uncontrolled seasonal allergic rhinitis (PHECDA): Rationale and design of a multicentre, randomized, double-blind, placebo-controlled study.Asia Pac Allergy. 2025 Mar;15(1):15-20. doi: 10.5415/apallergy.0000000000000174. Epub 2025 Jan 13. Asia Pac Allergy. 2025. PMID: 40051426 Free PMC article.
-
Stapokibart for Severe Uncontrolled Chronic Rhinosinusitis With Nasal Polyps: The CROWNS-2 Randomized Clinical Trial.JAMA. 2025 Aug 18:e2512515. doi: 10.1001/jama.2025.12515. Online ahead of print. JAMA. 2025. PMID: 40824573
-
Efficacy and safety of twice-daily olopatadine-mometasone combination nasal spray (GSP301) in the treatment of allergic rhinitis: a systematic review and meta-analysis.Eur Arch Otorhinolaryngol. 2022 Apr;279(4):1691-1699. doi: 10.1007/s00405-021-07085-w. Epub 2021 Sep 30. Eur Arch Otorhinolaryngol. 2022. PMID: 34591150
-
Evaluating stapokibart in the treatment of seasonal allergic rhinitis.Immunotherapy. 2025 Sep 3:1-10. doi: 10.1080/1750743X.2025.2554562. Online ahead of print. Immunotherapy. 2025. PMID: 40900141 Review.
Cited by
-
Advances in Biologic Therapies for Allergic Diseases: Current Trends, Emerging Agents, and Future Perspectives.J Clin Med. 2025 Feb 8;14(4):1079. doi: 10.3390/jcm14041079. J Clin Med. 2025. PMID: 40004611 Free PMC article. Review.
-
Stapokibart for moderate-to-severe seasonal allergic rhinitis: a randomized phase 3 trial.Nat Med. 2025 Jul;31(7):2213-2221. doi: 10.1038/s41591-025-03651-5. Epub 2025 Apr 4. Nat Med. 2025. PMID: 40186079 Free PMC article. Clinical Trial.
-
Long-Term Efficacy and Safety of Stapokibart in Adults with Moderate-to-Severe Atopic Dermatitis: An Open-Label Extension, Nonrandomized Clinical Trial.BioDrugs. 2024 Sep;38(5):681-689. doi: 10.1007/s40259-024-00668-z. Epub 2024 Jul 31. BioDrugs. 2024. PMID: 39080181 Clinical Trial.
-
Stapokibart (CM310) in patients with uncontrolled seasonal allergic rhinitis (PHECDA): Rationale and design of a multicentre, randomized, double-blind, placebo-controlled study.Asia Pac Allergy. 2025 Mar;15(1):15-20. doi: 10.5415/apallergy.0000000000000174. Epub 2025 Jan 13. Asia Pac Allergy. 2025. PMID: 40051426 Free PMC article.
-
Recent Studies and Prospects of Biologics in Allergic Rhinitis Treatment.Int J Mol Sci. 2025 May 9;26(10):4509. doi: 10.3390/ijms26104509. Int J Mol Sci. 2025. PMID: 40429652 Free PMC article. Review.
References
-
- Wise S.K., Damask C., Roland L.T., et al. International consensus statement on allergy and rhinology: allergic rhinitis - 2023. Int Forum Allergy Rhinol. 2023;13:293–859. - PubMed
-
- Zuberbier T., Lotvall J., Simoens S., Subramanian S.V., Church M.K. Economic burden of inadequate management of allergic diseases in the European Union: a GA(2) LEN review. Allergy. 2014;69:1275–1279. - PubMed
-
- Colas C., Brosa M., Anton E., et al. Estimate of the total costs of allergic rhinitis in specialized care based on real-world data: the FERIN Study. Allergy. 2017;72:959–966. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

