Spectroscopically Orthogonal Labelling to Disentangle Site-Specific Nitroxide Label Distributions
- PMID: 38357007
- PMCID: PMC10861635
- DOI: 10.1007/s00723-023-01611-1
Spectroscopically Orthogonal Labelling to Disentangle Site-Specific Nitroxide Label Distributions
Abstract
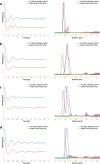
Biomolecular applications of pulse dipolar electron paramagnetic resonance spectroscopy (PDS) are becoming increasingly valuable in structural biology. Site-directed spin labelling of proteins is routinely performed using nitroxides, with paramagnetic metal ions and other organic radicals gaining popularity as alternative spin centres. Spectroscopically orthogonal spin labelling using different types of labels potentially increases the information content available from a single sample. When analysing experimental distance distributions between two nitroxide spin labels, the site-specific rotamer information has been projected into the distance and is not readily available, and the contributions of individual labelling sites to the width of the distance distribution are not obvious from the PDS data. Here, we exploit the exquisite precision of labelling double-histidine (dHis) motifs with CuII chelate complexes. The contribution of this label to the distance distribution widths in model protein GB1 has been shown to be negligible. By combining a dHis CuII labelling site with cysteine-specific nitroxide labelling, we gather insights on the label rotamers at two distinct sites, comparing their contributions to distance distributions based on different in silico modelling approaches and structural models. From this study, it seems advisable to consider discrepancies between different in silico modelling approaches when selecting labelling sites for PDS studies.
Supplementary information: The online version contains supplementary material available at 10.1007/s00723-023-01611-1.
© The Author(s) 2023.
Conflict of interest statement
Conflict of InterestAll authors declare that they have no conflict of interest.
Figures


References
-
- Edwards DT, Huber T, Hussain S, Stone KM, Kinnebrew M, Kaminker I, Matalon E, Sherwin MS, Goldfarb D, Han S. Determining the oligomeric structure of proteorhodopsin by Gd3+-based pulsed dipolar spectroscopy of multiple distances. Structure. 2014;22:1677–1686. doi: 10.1016/j.str.2014.09.008. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
