Updates in the pathogenesis and management of immune-related enterocolitis, hepatitis and cardiovascular toxicities
- PMID: 38357008
- PMCID: PMC10865026
- DOI: 10.1016/j.iotech.2024.100704
Updates in the pathogenesis and management of immune-related enterocolitis, hepatitis and cardiovascular toxicities
Abstract
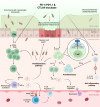
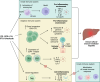
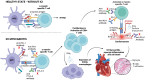
Immune checkpoint inhibitors (ICIs) have become a cornerstone of treatment for many solid organ malignancies. Alongside increasing use, the occurrence of immune-related adverse events (irAEs) has also increased and remains a significant challenge when treating patients with ICI. The underlying pathophysiology of irAE development for many organ systems is yet to be elucidated, but may involve unmasking of latent autoimmunity, increased T-cell recognition of shared antigens on cancer and normal tissue and ICI-triggered immune dysregulation with overactivation of proinflammatory pathways and suppression of immune control pathways. Management strategies for irAEs have historically been borrowed from paradigms for conventional autoimmune conditions such as inflammatory bowel disease and autoimmune hepatitis; however, recent translational efforts have clearly demonstrated key differences in underlying immune signalling pathways. As we begin to understand these differences, we must adapt a more targeted approach to immunosuppression and exercise a more nuanced approach with the multiple biologic agents available to mitigate ICI-related toxicity without reversing the antitumour effect of ICI. In this review, we focus on three key immune-related toxicities where recent clinical and translational work has provided nuanced insights into pathogenesis and treatment strategies: enterocolitis, hepatitis and cardiovascular toxicity including myocarditis.
Keywords: enterocolitis; hepatitis; immune-related adverse events; immunotherapy; myocarditis.
© 2024 The Authors.
Figures
References
-
- Gandhi L., Rodríguez-Abreu D., Gadgeel S., et al. Pembrolizumab plus chemotherapy in metastatic non–small-cell lung cancer. N Engl J Med. 2018;378(22):2078–2092. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

