Thrombopoietin receptor agonists use and risk of thrombotic events in patients with immune thrombocytopenic purpura: A systematic review and meta‑analysis of randomized controlled trials
- PMID: 38357229
- PMCID: PMC10865300
- DOI: 10.3892/br.2024.1732
Thrombopoietin receptor agonists use and risk of thrombotic events in patients with immune thrombocytopenic purpura: A systematic review and meta‑analysis of randomized controlled trials
Abstract
Thrombopoietin receptor agonists (TPO-RAs) have a role in second-line immune thrombocytopenic purpura (ITP) treatment, binding to and activating thrombopoietin receptors on megakaryocyte membranes in the bone marrow. This promotes megakaryocyte maturation and increases platelet production. Despite a 2-6% incidence of thrombotic events during TPO-RA treatment, it remains uncertain whether TPO-RAs elevate thrombosis rates. A comprehensive search of electronic databases was conducted using the relevant search criteria. To assess the risk of bias, the included studies were assessed using the revised Cochrane Risk of Bias Assessment Tool 2.0, and a meta-analysis was performed using RevMan 5.4.1. A total of 1,698 patients with ITP were included from randomized controlled trials (RCTs). There were 26 thromboembolic events in the TPO-RAs group and 4 in the control group. However, there was no significant difference in the incidence of thrombotic events between the two groups [odds ratio (OR)=1.76, 95% confidence interval (CI): 0.78-4.00, P=0.18], even if the duration of treatment was >12 weeks (OR=2.46, 95% CI: 0.81-7.43, P=0.11). Subgroup analysis showed that none of the four drugs significantly increased the incidence of thrombotic events (romiplostim: OR=0.92, 95% CI: 0.14-6.13, P=0.93; eltrombopag: OR=2.32, 95% CI: 0.64-8.47, P=0.20; avatrombopag: OR=4.15, 95% CI: 0.20-85.23, P=0.36; and hetrombopag: OR=0.76, 95% CI: 0.03-18.76, P=0.87). There was also no significant difference in the results of the double-blinded placebo-controlled RCTs (OR=1.21, 95% CI: 0.41-3.58, P=0.73). Compared to patients with ITP who did not receive TPO-RA treatment, those receiving TPO-RA treatment did not exhibit a significantly increased risk of thrombotic events.
Keywords: immune thrombocytopenia; meta-analysis; thrombopoietin receptor agonists; thrombosis.
Copyright: © Shen et al.
Conflict of interest statement
The authors declare that they have no competing interests.
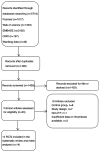
Figures





Similar articles
-
Safety of non‑peptide thrombopoietin receptor agonists in patients with immune thrombocytopenia: A systematic review and meta‑analysis of short‑term double‑blind randomized clinical trials.Exp Ther Med. 2023 Jul 3;26(2):393. doi: 10.3892/etm.2023.12092. eCollection 2023 Aug. Exp Ther Med. 2023. PMID: 37456173 Free PMC article.
-
Thrombopoietin receptor agonists for the treatment of primary immune thrombocytopenia: a meta-analysis and systematic review.Platelets. 2021 Feb 17;32(2):216-226. doi: 10.1080/09537104.2020.1745168. Epub 2020 Apr 12. Platelets. 2021. PMID: 32281449
-
Efficacy and safety of thrombopoietin receptor agonists in children with chronic immune thrombocytopenic purpura: meta-analysis.Platelets. 2019;30(7):828-835. doi: 10.1080/09537104.2019.1572873. Epub 2019 Feb 27. Platelets. 2019. PMID: 30810479
-
Treatment patterns and outcomes of second-line rituximab and thrombopoietin receptor agonists in adult immune thrombocytopenia: A Canadian retrospective cohort study.Thromb Res. 2022 Dec;220:5-11. doi: 10.1016/j.thromres.2022.09.021. Epub 2022 Sep 29. Thromb Res. 2022. PMID: 36257098
-
Efficacy and safety and analysis of thrombopoietin receptor agonists for the treatment of immune thrombocytopenia in adults: analysis of a systematic review and network meta-analysis of randomized controlled trials and results of real-world safety data.Front Med (Lausanne). 2025 Mar 11;12:1531824. doi: 10.3389/fmed.2025.1531824. eCollection 2025. Front Med (Lausanne). 2025. PMID: 40196346 Free PMC article.
Cited by
-
Analysis of Risk Factors and the Establishment of a Predictive Model for Thrombosis in Patients with Immune Thrombocytopenia.Clin Appl Thromb Hemost. 2025 Jan-Dec;31:10760296241301398. doi: 10.1177/10760296241301398. Clin Appl Thromb Hemost. 2025. PMID: 39763222 Free PMC article.
-
Insights on treatment of adult ITP: algorithm for management and role of multimodal therapy.Hematology Am Soc Hematol Educ Program. 2024 Dec 6;2024(1):678-684. doi: 10.1182/hematology.2024000594. Hematology Am Soc Hematol Educ Program. 2024. PMID: 39643995 Free PMC article. Review.
-
Prevalence and characteristics of acute ischemic stroke and intracranial hemorrhage in patients with immune thrombocytopenic purpura and immune thrombotic thrombocytopenic purpura: a systematic review and meta-analysis.Neurol Res Pract. 2025 Mar 17;7(1):19. doi: 10.1186/s42466-025-00374-3. Neurol Res Pract. 2025. PMID: 40091091 Free PMC article.
-
Relationship between thromboembolic events and thrombopoietin receptor agonists: a pharmacovigilance analysis of the FDA Adverse Event Reporting System and the Japanese Adverse Drug Event Report.BMJ Open. 2025 Aug 10;15(8):e099153. doi: 10.1136/bmjopen-2025-099153. BMJ Open. 2025. PMID: 40784768 Free PMC article.
-
Hetrombopag treatment for immune thrombocytopenia in pregnancy refractory to corticosteroids and intravenous immunoglobulin: a case report.Front Med (Lausanne). 2025 Feb 18;12:1528131. doi: 10.3389/fmed.2025.1528131. eCollection 2025. Front Med (Lausanne). 2025. PMID: 40041460 Free PMC article.
References
LinkOut - more resources
Full Text Sources
