Drug utilization studies in Turkiye: A systematic review
- PMID: 38357318
- PMCID: PMC10861429
- DOI: 10.14744/nci.2023.60134
Drug utilization studies in Turkiye: A systematic review
Abstract
Objective: Drug utilization studies (DUS) provide a framework for drug utilization at the national or targeted population level and important information on unmet medical needs, particularly in assessing the rationality of drug use. We aimed to systematically review DUS conducted in Turkiye.
Methods: We examined 162 DUS with an accessible full-text, published as "research articles" and conducted in Turkiye between 2000 and 2021 using medical records and prescription data. We included English or Turkish papers with English abstracts. We examined the scientific characteristics of the publications, source of the data, place/time of collection, research designs, and studied drug groups.
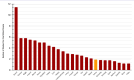
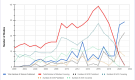
Results: We found that 79.6% of articles were in English, 45.1% were listed in SCI/SCIE, and 63.0% were on the WOS platform with 3.5 (interquartile range: 1-15) citations. The mean study period and publication time were 2.9±3.1 and 2.9±2.1 years, respectively. The highest number of studies (17.9%) were published in 2021 and (26.5%) were conducted nationwide. We identified that 93.8% of the studies had retrospective design, 67.8% were conducted in secondary/tertiary health-care institutions, and 54.9% used direct hospital data. We detected that 68.5% of the studies were conducted on the general population, 19.1% on adults, 12.4% on children, and 44.4% were antibiotic oriented.
Conclusion: Our study showed that a significant portion of the DUS, the trend of which has gained momentum in recent years, was antibiotic focused and conducted with a retrospective design from hospital-based data collected on the general patient population. This situation points to the necessity of expanding the existing DUS range by effectively using the new advantages provided by medical record databases and conducting more DUS that can provide critical clues for specific patients and drug groups.
Keywords: Drug utilization studies; pharmacoepidemiology; research; systematic review.
© Copyright 2024 by Istanbul Provincial Directorate of Health.
Conflict of interest statement
No conflict of interest was declared by the authors.
Figures
Similar articles
-
Systematic review of survey/questionnaire-based drug utilization studies in Turkiye.North Clin Istanb. 2024 Nov 22;11(6):525-533. doi: 10.14744/nci.2024.60252. eCollection 2024. North Clin Istanb. 2024. PMID: 39650312 Free PMC article.
-
Beyond the black stump: rapid reviews of health research issues affecting regional, rural and remote Australia.Med J Aust. 2020 Dec;213 Suppl 11:S3-S32.e1. doi: 10.5694/mja2.50881. Med J Aust. 2020. PMID: 33314144
-
Recovery schools for improving behavioral and academic outcomes among students in recovery from substance use disorders: a systematic review.Campbell Syst Rev. 2018 Oct 4;14(1):1-86. doi: 10.4073/csr.2018.9. eCollection 2018. Campbell Syst Rev. 2018. PMID: 37131375 Free PMC article.
-
The Nordic prescription databases as a resource for pharmacoepidemiological research--a literature review.Pharmacoepidemiol Drug Saf. 2013 Jul;22(7):691-9. doi: 10.1002/pds.3457. Epub 2013 May 23. Pharmacoepidemiol Drug Saf. 2013. PMID: 23703712 Review.
-
Reducing unplanned hospital admissions from care homes: a systematic review.Health Soc Care Deliv Res. 2023 Oct;11(18):1-130. doi: 10.3310/KLPW6338. Health Soc Care Deliv Res. 2023. PMID: 37916580
Cited by
-
Systematic review of survey/questionnaire-based drug utilization studies in Turkiye.North Clin Istanb. 2024 Nov 22;11(6):525-533. doi: 10.14744/nci.2024.60252. eCollection 2024. North Clin Istanb. 2024. PMID: 39650312 Free PMC article.
References
-
- Wettermark B, Elseviers M, Almarsdóttir AB, Andersen M, Benko R, Bennie M, at al. Introduction to drug utilization research. In: Elseviers M, Wettermark B, Almarsdóttir AB, Andersen M, Benko R, Bennie M, et al., editors. Drug Utilization Research: Methods and Applications. 1st ed. England: Wiley; 2016. pp. 3–14.
-
- Shalini S, Ravichandran V, Saraswathi R, Mohanty BK, Dhanaraj SK. Drug utilization studies–an overview. Int J Pharm Sci Nanotech. 2010;3:803–10.
-
- Sacristén JA, Soto J. Drug utilization studies as tools in health economics. Pharmacoeconomics. 1994;5:299–312. - PubMed
-
- Bergman U. The history of the Drug Utilization Research Group in Europe. Pharmacoepidemiol Drug Saf. 2006;15:95–8. - PubMed
-
- Duran CE, Christiaens T, Acosta A, Vander Stichele R. Systematic review of cross-national drug utilization studies in Latin America: methods and comparability. Pharmacoepidemiol Drug Saf. 2016;25:16–25. - PubMed
LinkOut - more resources
Full Text Sources
