Roles of Lipolytic enzymes in Mycobacterium tuberculosis pathogenesis
- PMID: 38357346
- PMCID: PMC10865251
- DOI: 10.3389/fmicb.2024.1329715
Roles of Lipolytic enzymes in Mycobacterium tuberculosis pathogenesis
Abstract
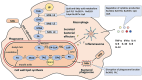
Mycobacterium tuberculosis (Mtb) is a bacterial pathogen that can endure for long periods in an infected patient, without causing disease. There are a number of virulence factors that increase its ability to invade the host. One of these factors is lipolytic enzymes, which play an important role in the pathogenic mechanism of Mtb. Bacterial lipolytic enzymes hydrolyze lipids in host cells, thereby releasing free fatty acids that are used as energy sources and building blocks for the synthesis of cell envelopes, in addition to regulating host immune responses. This review summarizes the relevant recent studies that used in vitro and in vivo models of infection, with particular emphasis on the virulence profile of lipolytic enzymes in Mtb. A better understanding of these enzymes will aid the development of new treatment strategies for TB. The recent work done that explored mycobacterial lipolytic enzymes and their involvement in virulence and pathogenicity was highlighted in this study. Lipolytic enzymes are expected to control Mtb and other intracellular pathogenic bacteria by targeting lipid metabolism. They are also potential candidates for the development of novel therapeutic agents.
Keywords: Mycobacterium tuberculosis; lipolytic enzymes; pathogenicity; therapeutic targets; virulence factor.
Copyright © 2024 Lin, Xing, Wang, Wang, Fang, Li, Li and Song.
Conflict of interest statement
ZL was employed by the SAFE Pharmaceutical Technology Co. Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Lipid metabolism and intracellular bacterial virulence: key to next-generation therapeutics.Future Microbiol. 2018 Sep;13:1301-1328. doi: 10.2217/fmb-2018-0013. Epub 2018 Sep 26. Future Microbiol. 2018. PMID: 30256124 Review.
-
Editorial: Current status and perspective on drug targets in tubercle bacilli and drug design of antituberculous agents based on structure-activity relationship.Curr Pharm Des. 2014;20(27):4305-6. doi: 10.2174/1381612819666131118203915. Curr Pharm Des. 2014. PMID: 24245755
-
Lipid hydrolizing enzymes in virulence: Mycobacterium tuberculosis as a model system.Crit Rev Microbiol. 2010 Aug;36(3):259-69. doi: 10.3109/1040841X.2010.482923. Crit Rev Microbiol. 2010. PMID: 20500016 Review.
-
Lipolytic enzymes in Mycobacterium tuberculosis.Appl Microbiol Biotechnol. 2008 Apr;78(5):741-9. doi: 10.1007/s00253-008-1397-2. Epub 2008 Feb 29. Appl Microbiol Biotechnol. 2008. PMID: 18309478 Review.
-
Mycobacterium tuberculosis Rv1324 Protein Contributes to Mycobacterial Persistence and Causes Pathological Lung Injury in Mice by Inducing Ferroptosis.Microbiol Spectr. 2023 Feb 14;11(1):e0252622. doi: 10.1128/spectrum.02526-22. Epub 2023 Jan 10. Microbiol Spectr. 2023. PMID: 36625672 Free PMC article.
Cited by
-
Diabetes and tuberculosis: An emerging dual threat to healthcare.World J Diabetes. 2024 Jul 15;15(7):1409-1416. doi: 10.4239/wjd.v15.i7.1409. World J Diabetes. 2024. PMID: 39099826 Free PMC article.
-
Proteomic characterization of Mycobacterium tuberculosis subjected to carbon starvation.mSystems. 2025 May 20;10(5):e0153024. doi: 10.1128/msystems.01530-24. Epub 2025 Apr 15. mSystems. 2025. PMID: 40231840 Free PMC article.
References
-
- Anand P. K., Kaur J. (2023). Rv3539 (PPE63) of Mycobacterium tuberculosis promotes survival of Mycobacterium smegmatis in human macrophages cell line via cell wall modulation of bacteria and altering host’s immune response. Curr. Microbiol. 80:267. doi: 10.1007/s00284-023-03360-7, PMID: - DOI - PubMed
-
- Arya S., Singh P., Kaur J., Kumar A., Kaur J. (2022). Environment dependent expression of mycobacterium hormone sensitive lipases: expression pattern under ex-vivo and individual in-vitro stress conditions in M. tuberculosis H37Ra. Mol. Biol. Rep. 49, 4583–4593. doi: 10.1007/s11033-022-07305-4 - DOI - PubMed
-
- Bacon J., Dover L. G., Hatch K. A., Zhang Y., Gomes J. M., Kendall S., et al. . (2007). Lipid composition and transcriptional response of Mycobacterium tuberculosis grown under iron-limitation in continuous culture: identification of a novel wax ester. Microbiology 153, 1435–1444. doi: 10.1099/mic.0.2006/004317-0 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

