Synthesis and Characterization of Palladium Pincer Bis(carbene) CCC Complexes
- PMID: 38357387
- PMCID: PMC10863395
- DOI: 10.1021/acs.organomet.3c00114
Synthesis and Characterization of Palladium Pincer Bis(carbene) CCC Complexes
Abstract
The metalation of the DIPPCCC (DIPPCCC = bis(diisopropylphenyl-imidazol-2-ylidene)phenyl) ligand platform with Pd was achieved under mild conditions by reacting [H3(DIPPCCC)]Cl2 with Pd(OAc)2 at room temperature in the presence of 3.1 equiv of LiN(SiMe3)2. The resulting complexes (DIPPCCC)PdX (X = Cl or Br) were oxidized by two-electron oxidants PhICl2, Br2, and BTMABr3. All the complexes were crystallographically characterized, and analysis of structural parameters around the ligand scaffold show no evidence of a ligand-centered radical, rendering the metal center in the oxidized species, (DIPPCCC)PdX3 (X = Cl or Br), a formal PdIV oxidation state. Unlike their NiIV analogues, these PdIV complexes are stable to air and moisture. The addition of styrene to (DIPPCCC)PdBr3 resulted in the clean reduction of PdIV to PdII, along with the formation of the halogenated alkane. The oxidation to PdIV and subsequent return to PdII upon reduction, as opposed to formation of PdIII species, showcases the accessibility of high-valent palladium DIPPCCC complexes.
© 2023 American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




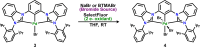
References
-
- González-Sebastián L.; Morales-Morales D. Cross-Coupling Reactions Catalysed by Palladium Pincer Complexes. A Review of Recent Advances. J. Organomet. Chem. 2019, 893, 39–51. 10.1016/j.jorganchem.2019.04.021. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous
