Risk of Arterial and Venous Thrombotic Events Among Patients with COVID-19: A Multi-National Collaboration of Regulatory Agencies from Canada, Europe, and United States
- PMID: 38357585
- PMCID: PMC10865892
- DOI: 10.2147/CLEP.S448980
Risk of Arterial and Venous Thrombotic Events Among Patients with COVID-19: A Multi-National Collaboration of Regulatory Agencies from Canada, Europe, and United States
Abstract
Purpose: Few studies have examined how the absolute risk of thromboembolism with COVID-19 has evolved over time across different countries. Researchers from the European Medicines Agency, Health Canada, and the United States (US) Food and Drug Administration established a collaboration to evaluate the absolute risk of arterial (ATE) and venous thromboembolism (VTE) in the 90 days after diagnosis of COVID-19 in the ambulatory (eg, outpatient, emergency department, nursing facility) setting from seven countries across North America (Canada, US) and Europe (England, Germany, Italy, Netherlands, and Spain) within periods before and during COVID-19 vaccine availability.
Patients and methods: We conducted cohort studies of patients initially diagnosed with COVID-19 in the ambulatory setting from the seven specified countries. Patients were followed for 90 days after COVID-19 diagnosis. The primary outcomes were ATE and VTE over 90 days from diagnosis date. We measured country-level estimates of 90-day absolute risk (with 95% confidence intervals) of ATE and VTE.
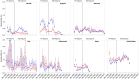
Results: The seven cohorts included 1,061,565 patients initially diagnosed with COVID-19 in the ambulatory setting before COVID-19 vaccines were available (through November 2020). The 90-day absolute risk of ATE during this period ranged from 0.11% (0.09-0.13%) in Canada to 1.01% (0.97-1.05%) in the US, and the 90-day absolute risk of VTE ranged from 0.23% (0.21-0.26%) in Canada to 0.84% (0.80-0.89%) in England. The seven cohorts included 3,544,062 patients with COVID-19 during vaccine availability (beginning December 2020). The 90-day absolute risk of ATE during this period ranged from 0.06% (0.06-0.07%) in England to 1.04% (1.01-1.06%) in the US, and the 90-day absolute risk of VTE ranged from 0.25% (0.24-0.26%) in England to 1.02% (0.99-1.04%) in the US.
Conclusion: There was heterogeneity by country in 90-day absolute risk of ATE and VTE after ambulatory COVID-19 diagnosis both before and during COVID-19 vaccine availability.
Keywords: COVID-19; ischemic stroke; myocardial infarction; thromboembolism; venous thromboembolism.
© 2024 Lo Re III et al.
Conflict of interest statement
VLR III reports research grants to his institution from the US Food and Drug Administration (FDA) and the National Institutes of Health (NIH); consulting fees from Entasis, Takeda, and Urovant Sciences; and participation on the FDA Drug Safety and Risk Management Advisory Committee. NMC reports research funding from FDA via a Department of Health and Human Services (HHS) contract during the conduct of the study and funding from HPHCI (a non-profit organization that conducts work for government and private organizations, including pharmaceutical companies) outside the submitted work. RAH reports grants from FDA, NIH, and Merck. AMP reports royalties from UpToDate (including for an article on anticoagulation), consulting fees via advisory board from BioMarin LLC outside the submitted work, and speaker honorarium from the American Society of Hematology. DAD reports stock options in CVS Health. JLK reports unrelated research grants to her institution from Vir Biotechnology, Pfizer, Novartis, and the Centers for Disease Control and Prevention. PRR reports unrelated research grants to his institution from Chiesi, UCB, Amgen, Johnson & Johnson, Innovative Medicines Initiative, and the European Medicines Agency. KV reports unrelated research grants to her institution from Chiesi, UCB, Amgen, Johnson & Johnson, and the European Medicines Agency. DPA reports research grants to his institution from Amgen, Chiesi-Taylor, Lilly, Janssen, Novartis, and UCB Biopharma as well as consulting fees from Astra Zeneca and UCB Biopharma. The authors report no other conflicts of interest in this work.
Figures
References
LinkOut - more resources
Full Text Sources
Research Materials

