Injectable Granular Hydrogels Enable Avidity-Controlled Biotherapeutic Delivery
- PMID: 38357739
- PMCID: PMC10934254
- DOI: 10.1021/acsbiomaterials.3c01906
Injectable Granular Hydrogels Enable Avidity-Controlled Biotherapeutic Delivery
Abstract
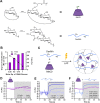
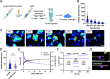
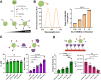
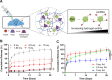
Protein therapeutics represent a rapidly growing class of pharmaceutical agents that hold great promise for the treatment of various diseases such as cancer and autoimmune dysfunction. Conventional systemic delivery approaches, however, result in off-target drug exposure and a short therapeutic half-life, highlighting the need for more localized and controlled delivery. We have developed an affinity-based protein delivery system that uses guest-host complexation between β-cyclodextrin (CD, host) and adamantane (Ad, guest) to enable sustained localized biomolecule presentation. Hydrogels were formed by the copolymerization of methacrylated CD and methacrylated dextran. Extrusion fragmentation of bulk hydrogels yielded shear-thinning and self-healing granular hydrogels (particle diameter = 32.4 ± 16.4 μm) suitable for minimally invasive delivery and with a high host capacity for the retention of guest-modified proteins. Bovine serum albumin (BSA) was controllably conjugated to Ad via EDC chemistry without affecting the affinity of the Ad moiety for CD (KD = 12.0 ± 1.81 μM; isothermal titration calorimetry). The avidity of Ad-BSA conjugates was directly tunable through the number of guest groups attached, resulting in a fourfold increase in the complex half-life (t1/2 = 5.07 ± 1.23 h, surface plasmon resonance) that enabled a fivefold reduction in protein release at 28 days. Furthermore, we demonstrated that the conjugation of Ad to immunomodulatory cytokines (IL-4, IL-10, and IFNγ) did not detrimentally affect cytokine bioactivity and enabled their sustained release. Our strategy of avidity-controlled delivery of protein-based therapeutics is a promising approach for the sustained local presentation of protein therapeutics and can be applied to numerous biomedical applications.
Keywords: avidity; bioconjugation; cyclodextrin; hydrogel; sustained release.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
Similar articles
-
Rational design of network properties in guest-host assembled and shear-thinning hyaluronic acid hydrogels.Biomacromolecules. 2013 Nov 11;14(11):4125-34. doi: 10.1021/bm401280z. Epub 2013 Oct 14. Biomacromolecules. 2013. PMID: 24070551 Free PMC article.
-
A supramolecular host-guest interaction-mediated injectable hydrogel system with enhanced stability and sustained protein release.Acta Biomater. 2021 Sep 1;131:286-301. doi: 10.1016/j.actbio.2021.07.004. Epub 2021 Jul 8. Acta Biomater. 2021. PMID: 34246803
-
Sustained Small Molecule Delivery from Injectable Hyaluronic Acid Hydrogels through Host-Guest Mediated Retention.J Mater Chem B. 2015 Oct 28;3(40):8010-8019. doi: 10.1039/C5TB00981B. Epub 2015 Sep 18. J Mater Chem B. 2015. PMID: 26693019 Free PMC article.
-
Designing Hydrogels for On-Demand Therapy.Acc Chem Res. 2017 Apr 18;50(4):669-679. doi: 10.1021/acs.accounts.6b00536. Epub 2017 Mar 16. Acc Chem Res. 2017. PMID: 28301139 Free PMC article. Review.
-
Injectable hydrogels for delivering biotherapeutic molecules.Int J Biol Macromol. 2018 Apr 15;110:17-29. doi: 10.1016/j.ijbiomac.2017.11.113. Epub 2017 Nov 21. Int J Biol Macromol. 2018. PMID: 29169942 Review.
Cited by
-
Granular hydrogels as brittle yield stress fluids.bioRxiv [Preprint]. 2025 Feb 27:2025.02.22.639638. doi: 10.1101/2025.02.22.639638. bioRxiv. 2025. Update in: Adv Mater. 2025 Jul 9:e2503635. doi: 10.1002/adma.202503635. PMID: 40060491 Free PMC article. Updated. Preprint.
-
Cardiac Matrix-Derived Granular Hydrogel Enhances Cell Function in 3D Culture.ACS Appl Mater Interfaces. 2024 Oct 30;16(43):58346-58356. doi: 10.1021/acsami.4c12871. Epub 2024 Oct 16. ACS Appl Mater Interfaces. 2024. PMID: 39413287 Free PMC article.
-
Lab on a Particle Technologies.Anal Chem. 2024 May 21;96(20):7817-7839. doi: 10.1021/acs.analchem.4c01510. Epub 2024 Apr 22. Anal Chem. 2024. PMID: 38650433 Free PMC article. Review. No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
