Directed Differentiation of Human Induced Pluripotent Stem Cells to Heart Valve Cells
- PMID: 38357822
- PMCID: PMC11062615
- DOI: 10.1161/CIRCULATIONAHA.123.065143
Directed Differentiation of Human Induced Pluripotent Stem Cells to Heart Valve Cells
Abstract
Background: A main obstacle in current valvular heart disease research is the lack of high-quality homogeneous functional heart valve cells. Human induced pluripotent stem cells (hiPSCs)-derived heart valve cells may help with this dilemma. However, there are no well-established protocols to induce hiPSCs to differentiate into functional heart valve cells, and the networks that mediate the differentiation have not been fully elucidated.
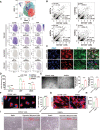
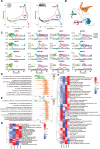
Methods: To generate heart valve cells from hiPSCs, we sequentially activated the Wnt, BMP4, VEGF (vascular endothelial growth factor), and NFATc1 signaling pathways using CHIR-99021, BMP4, VEGF-165, and forskolin, respectively. The transcriptional and functional similarity of hiPSC-derived heart valve cells compared with primary heart valve cells were characterized. Longitudinal single-cell RNA sequencing was used to uncover the trajectory, switch genes, pathways, and transcription factors of the differentiation.
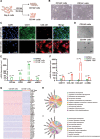
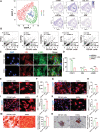
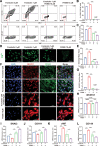
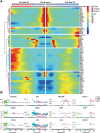
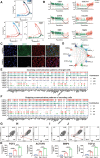
Results: An efficient protocol was developed to induce hiPSCs to differentiate into functional hiPSC-derived valve endothelial-like cells and hiPSC-derived valve interstitial-like cells. After 6-day differentiation and CD144 magnetic bead sorting, ≈70% CD144+ cells and 30% CD144- cells were obtained. On the basis of single-cell RNA sequencing data, the CD144+ cells and CD144- cells were found to be highly similar to primary heart valve endothelial cells and primary heart valve interstitial cells in gene expression profile. Furthermore, CD144+ cells had the typical function of primary heart valve endothelial cells, including tube formation, uptake of low-density lipoprotein, generation of endothelial nitric oxide synthase, and response to shear stress. Meanwhile, CD144- cells could secret collagen and matrix metalloproteinases, and differentiate into osteogenic or adipogenic lineages like primary heart valve interstitial cells. Therefore, we identified CD144+ cells and CD144- cells as hiPSC-derived valve endothelial-like cells and hiPSC-derived valve interstitial-like cells, respectively. Using single-cell RNA sequencing analysis, we demonstrated that the trajectory of heart valve cell differentiation was consistent with embryonic valve development. We identified the main switch genes (NOTCH1, HEY1, and MEF2C), signaling pathways (TGF-β, Wnt, and NOTCH), and transcription factors (MSX1, SP5, and MECOM) that mediated the differentiation. Finally, we found that hiPSC-derived valve interstitial-like cells might derive from hiPSC-derived valve endothelial-like cells undergoing endocardial-mesenchymal transition.
Conclusions: In summary, this is the first study to report an efficient strategy to generate functional hiPSC-derived valve endothelial-like cells and hiPSC-derived valve interstitial-like cells from hiPSCs, as well as to elucidate the differentiation trajectory and transcriptional dynamics of hiPSCs differentiated into heart valve cells.
Keywords: cell differentiation; endothelial cells; heart valves; induced pluripotent stem cells; single-cell gene expression analysis.
Conflict of interest statement
Figures

Comment in
-
Empowering Valvular Heart Disease Research With Stem Cell-Derived Valve Cells.Circulation. 2024 Apr 30;149(18):1457-1460. doi: 10.1161/CIRCULATIONAHA.124.068656. Epub 2024 Apr 29. Circulation. 2024. PMID: 38683900 Free PMC article. No abstract available.
-
Efficient generation of functional heart valve cells.Nat Cardiovasc Res. 2024 Mar;3(3):263. doi: 10.1038/s44161-024-00455-7. Nat Cardiovasc Res. 2024. PMID: 39196124 No abstract available.
References
-
- Yadgir S, Johnson CO, Aboyans V, Adebayo OM, Adedoyin RA, Afarideh M, Alahdab F, Alashi A, Alipour V, Arabloo J, et al. ; Global Burden of Disease Study 2017 Nonrheumatic Valve Disease Collaborators. Global, regional, and national burden of calcific aortic valve and degenerative mitral valve diseases, 1990–2017. Circulation. 2020;141:1670–1680. doi: 10.1161/CIRCULATIONAHA.119.043391 - PubMed
-
- Coffey S, Cox B, Williams MJ. Lack of progress in valvular heart disease in the pre-transcatheter aortic valve replacement era: increasing deaths and minimal change in mortality rate over the past three decades. Am Heart J. 2014;167:562–567.e2. doi: 10.1016/j.ahj.2013.12.030 - PubMed
-
- Coffey S, Roberts-Thomson R, Brown A, Carapetis J, Chen M, Enriquez-Sarano M, Zühlke L, Prendergast BD. Global epidemiology of valvular heart disease. Nat Rev Cardiol. 2021;18:853–864. doi: 10.1038/s41569-021-00570-z - PubMed
-
- Tsao CW, Aday AW, Almarzooq ZI, Alonso A, Beaton AZ, Bittencourt MS, Boehme AK, Buxton AE, Carson AP, Commodore-Mensah Y, et al. . Heart disease and stroke statistics-2022 update: a report from the American Heart Association. Circulation. 2022;145:e153–e639. doi: 10.1161/CIR.0000000000001052 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

