Carving out a Glycoside Hydrolase Active Site for Incorporation into a New Protein Scaffold Using Deep Network Hallucination
- PMID: 38357862
- PMCID: PMC10949244
- DOI: 10.1021/acssynbio.3c00674
Carving out a Glycoside Hydrolase Active Site for Incorporation into a New Protein Scaffold Using Deep Network Hallucination
Abstract
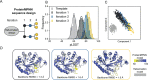
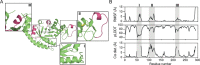
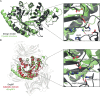
Enzymes are indispensable biocatalysts for numerous industrial applications, yet stability, selectivity, and restricted substrate recognition present limitations for their use. Despite the importance of enzyme engineering in overcoming these limitations, success is often challenged by the intricate architecture of enzymes derived from natural sources. Recent advances in computational methods have enabled the de novo design of simplified scaffolds with specific functional sites. Such scaffolds may be advantageous as platforms for enzyme engineering. Here, we present a strategy for the de novo design of a simplified scaffold of an endo-α-N-acetylgalactosaminidase active site, a glycoside hydrolase from the GH101 enzyme family. Using a combination of trRosetta hallucination, iterative cycles of deep-learning-based structure prediction, and ProteinMPNN sequence design, we designed proteins with 290 amino acids incorporating the active site while reducing the molecular weight by over 100 kDa compared to the initial endo-α-N-acetylgalactosaminidase. Of 11 tested designs, six were expressed as soluble monomers, displaying similar or increased thermostabilities compared to the natural enzyme. Despite lacking detectable enzymatic activity, the experimentally determined crystal structures of a representative design closely matched the design with a root-mean-square deviation of 1.0 Å, with most catalytically important side chains within 2.0 Å. The results highlight the potential of scaffold hallucination in designing proteins that may serve as a foundation for subsequent enzyme engineering.
Keywords: de novo design; deep network hallucination; enzyme design; glycoside hydrolase.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





References
-
- Leveson-Gower R. B.; Mayer C.; Roelfes G. The Importance of Catalytic Promiscuity for Enzyme Design and Evolution. Nat. Rev. Chem 2019, 3, 687–705. 10.1038/s41570-019-0143-x. - DOI
-
- Savile C. K.; Janey J. M.; Mundorff E. C.; Moore J. C.; Tam S.; Jarvis W. R.; Colbeck J. C.; Krebber A.; Fleitz F. J.; Brands J.; Devine P. N.; Huisman G. W.; Hughes G. J. Biocatalytic Asymmetric Synthesis of Chiral Amines from Ketones Applied to Sitagliptin Manufacture. Science 2010, 329 (5989), 305–309. 10.1126/science.1188934. - DOI - PubMed
-
- Huffman M. A.; Fryszkowska A.; Alvizo O.; Borra-Garske M.; Campos K. R.; Canada K. A.; Devine P. N.; Duan D.; Forstater J. H.; Grosser S. T.; Halsey H. M.; Hughes G. J.; Jo J.; Joyce L. A.; Kolev J. N.; Liang J.; Maloney K. M.; Mann B. F.; Marshall N. M.; McLaughlin M.; Moore J. C.; Murphy G. S.; Nawrat C. C.; Nazor J.; Novick S.; Patel N. R.; Rodriguez-Granillo A.; Robaire S. A.; Sherer E. C.; Truppo M. D.; Whittaker A. M.; Verma D.; Xiao L.; Xu Y.; Yang H. Design of an in Vitro Biocatalytic Cascade for the Manufacture of Islatravir. Science 2019, 366 (6470), 1255–1259. 10.1126/science.aay8484. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

