Loss of Endothelial Annexin A1 Aggravates Inflammation-Induched Vascular Aging
- PMID: 38358087
- PMCID: PMC11022713
- DOI: 10.1002/advs.202307040
Loss of Endothelial Annexin A1 Aggravates Inflammation-Induched Vascular Aging
Abstract
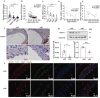
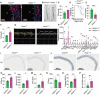
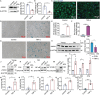
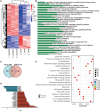
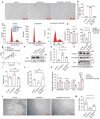
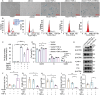
Chronic inflammation is increasingly considered as the most important component of vascular aging, contributing to the progression of age-related cardiovascular diseases. To delay the process of vascular aging, anti-inflammation may be an effective measure. The anti-inflammatory factor annexin A1 (ANXA1) is shown to participate in several age-related diseases; however, its function during vascular aging remains unclear. Here, an ANXA1 knockout (ANXA1-/-) and an endothelial cell-specific ANXA1 deletion mouse (ANXA1△EC) model are used to investigate the role of ANXA1 in vascular aging. ANXA1 depletion exacerbates vascular remodeling and dysfunction while upregulates age- and inflammation-related protein expression. Conversely, Ac2-26 (a mimetic peptide of ANXA1) supplementation reverses this phenomenon. Furthermore, long-term tumor necrosis factor-alpha (TNF-α) induction of human umbilical vein endothelial cells (HUVECs) increases cell senescence. Finally, the senescence-associated secretory phenotype and senescence-related protein expression, rates of senescence-β-galactosidase positivity, cell cycle arrest, cell migration, and tube formation ability are observed in both ANXA1-knockdown HUVECs and overexpressed ANXA1-TNF-α induced senescent HUVECs. They also explore the impact of formyl peptide receptor 2 (a receptor of ANXA1) in an ANXA1 overexpression inflammatory model. These data provide compelling evidence that age-related inflammation in arteries contributes to senescent endothelial cells that promote vascular aging.
Keywords: annexin A1; endothelial cell; inflammaging; vascular aging.
© 2024 The Authors. Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Ke Y., Li D., Zhao M., Liu C., Liu J., Zeng A., Shi X., Cheng S., Pan B., Zheng L., Hong H., Free Radical Biol. Med. 2018, 116, 88. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
