The unique salt bridge network in GlacPETase: a key to its stability
- PMID: 38358247
- PMCID: PMC10952487
- DOI: 10.1128/aem.02242-23
The unique salt bridge network in GlacPETase: a key to its stability
Abstract
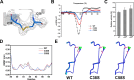
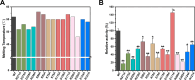
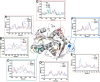
The extensive accumulation of polyethylene terephthalate (PET) has become a critical environmental issue. PET hydrolases can break down PET into its building blocks. Recently, we identified a glacial PET hydrolase GlacPETase sharing less than 31% amino acid identity with any known PET hydrolases. In this study, the crystal structure of GlacPETase was determined at 1.8 Å resolution, revealing unique structural features including a distinctive N-terminal disulfide bond and a specific salt bridge network. Site-directed mutagenesis demonstrated that the disruption of the N-terminal disulfide bond did not reduce GlacPETase's thermostability or its catalytic activity on PET. However, mutations in the salt bridges resulted in changes in melting temperature ranging from -8°C to +2°C and the activity on PET ranging from 17.5% to 145.5% compared to the wild type. Molecular dynamics simulations revealed that these salt bridges stabilized the GlacPETase's structure by maintaining their surrounding structure. Phylogenetic analysis indicated that GlacPETase represented a distinct branch within PET hydrolases-like proteins, with the salt bridges and disulfide bonds in this branch being relatively conserved. This research contributed to the improvement of our comprehension of the structural mechanisms that dictate the thermostability of PET hydrolases, highlighting the diverse characteristics and adaptability observed within PET hydrolases.IMPORTANCEThe pervasive problem of polyethylene terephthalate (PET) pollution in various terrestrial and marine environments is widely acknowledged and continues to escalate. PET hydrolases, such as GlacPETase in this study, offered a solution for breaking down PET. Its unique origin and less than 31% identity with any known PET hydrolases have driven us to resolve its structure. Here, we report the correlation between its unique structure and biochemical properties, focusing on an N-terminal disulfide bond and specific salt bridges. Through site-directed mutagenesis experiments and molecular dynamics simulations, the roles of the N-terminal disulfide bond and salt bridges were elucidated in GlacPETase. This research enhanced our understanding of the role of salt bridges in the thermostability of PET hydrolases, providing a valuable reference for the future engineering of PET hydrolases.
Keywords: PET hydrolase; molecular dynamics; salt bridges; structure; thermostability.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Glacier as a source of novel polyethylene terephthalate hydrolases.Environ Microbiol. 2023 Dec;25(12):2822-2833. doi: 10.1111/1462-2920.16516. Epub 2023 Sep 29. Environ Microbiol. 2023. PMID: 37775503
-
Computation-Based Design of Salt Bridges in PETase for Enhanced Thermostability and Performance for PET Degradation.Chembiochem. 2023 Nov 2;24(21):e202300373. doi: 10.1002/cbic.202300373. Epub 2023 Sep 13. Chembiochem. 2023. PMID: 37639367
-
Rational Design of Disulfide Bridges in BbPETaseCD for Enhancing the Enzymatic Performance in PET Degradation.Molecules. 2023 Apr 17;28(8):3528. doi: 10.3390/molecules28083528. Molecules. 2023. PMID: 37110762 Free PMC article.
-
Emerging Strategies in Polyethylene Terephthalate Hydrolase Research for Biorecycling.ChemSusChem. 2021 Oct 5;14(19):4115-4122. doi: 10.1002/cssc.202100740. Epub 2021 Jun 1. ChemSusChem. 2021. PMID: 33949146 Review.
-
[Advances in poly(ethylene terephthalate) hydrolases].Sheng Wu Gong Cheng Xue Bao. 2023 May 25;39(5):1998-2014. doi: 10.13345/j.cjb.220915. Sheng Wu Gong Cheng Xue Bao. 2023. PMID: 37212227 Review. Chinese.
Cited by
-
Biodegradation: the best solution to the world problem of discarded polymers.Bioresour Bioprocess. 2024 Aug 7;11(1):79. doi: 10.1186/s40643-024-00793-1. Bioresour Bioprocess. 2024. PMID: 39110313 Free PMC article. Review.
-
Atomistic-Level Insights into the Role of Mutations in the Engineering of PET Hydrolases: A Systematic Review.Int J Mol Sci. 2025 Aug 8;26(16):7682. doi: 10.3390/ijms26167682. Int J Mol Sci. 2025. PMID: 40869005 Free PMC article.
References
-
- PlasticsEurope . 2021. Plastics – the facts 2021: an analysis of European plastics production, demand and waste data
-
- Kawai F, Kawabata T, Oda M. 2020. Current state and perspectives related to the polyethylene terephthalate hydrolases available for biorecycling. ACS Sustainable Chem Eng 8:8894–8908. doi:10.1021/acssuschemeng.0c01638 - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

