VPS13C regulates phospho-Rab10-mediated lysosomal function in human dopaminergic neurons
- PMID: 38358348
- PMCID: PMC10868123
- DOI: 10.1083/jcb.202304042
VPS13C regulates phospho-Rab10-mediated lysosomal function in human dopaminergic neurons
Abstract
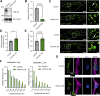
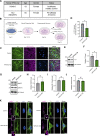
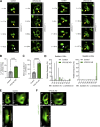
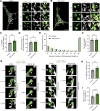
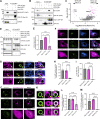
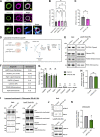
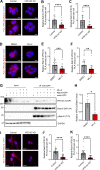
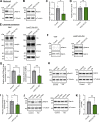
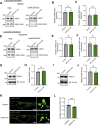
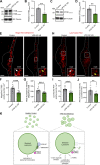
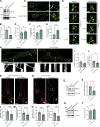
Loss-of-function mutations in VPS13C are linked to early-onset Parkinson's disease (PD). While VPS13C has been previously studied in non-neuronal cells, the neuronal role of VPS13C in disease-relevant human dopaminergic neurons has not been elucidated. Using live-cell microscopy, we investigated the role of VPS13C in regulating lysosomal dynamics and function in human iPSC-derived dopaminergic neurons. Loss of VPS13C in dopaminergic neurons disrupts lysosomal morphology and dynamics with increased inter-lysosomal contacts, leading to impaired lysosomal motility and cellular distribution, as well as defective lysosomal hydrolytic activity and acidification. We identified Rab10 as a phospho-dependent interactor of VPS13C on lysosomes and observed a decreased phospho-Rab10-mediated lysosomal stress response upon loss of VPS13C. These findings highlight an important role of VPS13C in regulating lysosomal homeostasis in human dopaminergic neurons and suggest that disruptions in Rab10-mediated lysosomal stress response contribute to disease pathogenesis in VPS13C-linked PD.
© 2024 Schröder et al.
Conflict of interest statement
Disclosures: D. Krainc is the founder and scientific advisory board chair of Vanqua Bio; serves on the scientific advisory boards of The Silverstein Foundation, Intellia Therapeutics, and AcureX Therapeutics; and is a venture partner at OrbiMed. No other disclosures were reported.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

