Total Synthesis of Dragocins A-C through Electrochemical Cyclization
- PMID: 38358802
- PMCID: PMC11619770
- DOI: 10.1002/anie.202401107
Total Synthesis of Dragocins A-C through Electrochemical Cyclization
Abstract
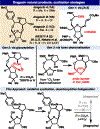
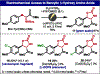
The first total synthesis of dragocins A-C, remarkable natural products containing an unusual C4' oxidized ribose architecture bridged by a polyhydroxylated pyrrolidine, is presented through a route featuring a number of uncommon maneuvers. Several generations towards the target molecules are presented, including the spectacular failure of a key C-H oxidation on a late-stage intermediate. The final route features rapid, stereocontrolled access to a densely functionalized pyrrolidine and an unprecedented diastereoselective oxidative electrochemical cyclization to forge the hallmark 9-membered ring. Preliminary studies suggest this electrochemical oxidation protocol is generally useful.
Keywords: alkaloids; electrochemistry; total synthesis.
© 2024 Wiley‐VCH GmbH.
Figures

References
-
- Choi H, Engene N, Byrum T, Hwang S, Oh DC, Gerwick WH, Org. Lett. 2019, 21, 266–270. - PubMed
-
- Kanbe K, Mimura Y, Tamamura T, Yatagai S, Sato Y, Takahashi A, Sato K, Naganawa H, Nakamura H, Takeuchi T, et al. , J. Antibiot. 1992, 45, 458–464. - PubMed
-
- Nakata M, Tamai T, Kamio T, Kinoshita M, Tatsuta K, Bull. Chem. Soc. Jpn 1994, 67, 3057–3066;
- Nakata M, Tamai T, Kamio T, Kinoshita M, Tatsuta K, Tetrahedron Lett. 1994, 35, 3099–3102.
-
- Thomas SO, Singleton VL, Lowery JA, Sharpe RW, Pruess LM, Porter JN, Mowat JH, Bohonos N, Antibiot. Annu. 1956, 716–721. - PubMed
-
- Reyes RL, Iwai T, Sawamura M, Chem. Rev. 2021, 121, 8926–8947; - PubMed
- Kleinke AS, Webb D, Jamison TF, Tetrahedron 2012, 68, 6999–7018;
- Illuminati G, Mandolini L, Acc. Chem. Res. 1981, 14, 95–102.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

