Development and Validation of a Prediction Model of Outcome After B-Cell Maturation Antigen-Directed Chimeric Antigen Receptor T-Cell Therapy in Relapsed/Refractory Multiple Myeloma
- PMID: 38358946
- PMCID: PMC11095856
- DOI: 10.1200/JCO.23.02232
Development and Validation of a Prediction Model of Outcome After B-Cell Maturation Antigen-Directed Chimeric Antigen Receptor T-Cell Therapy in Relapsed/Refractory Multiple Myeloma
Abstract
Purpose: Although chimeric antigen receptor T therapy (CAR-T) cells are an established therapy for relapsed/refractory multiple myeloma (RRMM), there are no established models predicting outcome to identify patients who may benefit the most from CAR-T.
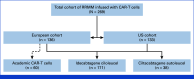
Patients and methods: This is an international retrospective observational study including patients with RRMM infused with currently available commercial or academically produced anti-B-cell maturation antigen (BCMA) CAR-T. We describe characteristics and outcomes in Europe (n = 136) and the United States (n = 133). Independent predictors of relapse/progression built a simple prediction model (Myeloma CAR-T Relapse [MyCARe] model) in the training cohort (Europe), which was externally validated (US cohort) and tested within patient- and treatment-specific subgroups.
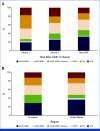
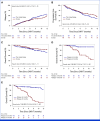
Results: The overall response rate was 87% and comparable between both cohorts, and complete responses were seen in 48% (Europe) and 49% (the United States). The median time to relapse was 5 months, and early relapse <5 months from infusion showed poor survival across cohorts, with the 12-month overall survival of 30% (Europe) and 14% (the United States). The presence of extramedullary disease or plasma cell leukemia, lenalidomide-refractoriness, high-risk cytogenetics, and increased ferritin at the time of lymphodepletion were independent predictors of early relapse or progression. Each factor received one point, forming the three-tiered MyCARe model: scores 0-1 (low risk), scores 2-3 (intermediate risk), and a score of 4 (high risk). The MyCARe model was significantly associated with distinct 5-month incidence of relapse/progression (P < .001): 7% for low-risk, 27% for intermediate-risk, and 53% for high-risk groups. The model was validated in the US cohort and maintained prognostic utility for response, survival, and outcomes across subgroups.
Conclusion: Outcomes of patients with RRMM after CAR-T are comparable between Europe and the United States. The MyCARe model may facilitate optimal timing of CAR-T cells in patient-specific subgroups.
Conflict of interest statement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (
Figures

References
-
- Berdeja JG, Madduri D, Usmani SZ, et al. Ciltacabtagene autoleucel, a B-cell maturation antigen-directed chimeric antigen receptor T-cell therapy in patients with relapsed or refractory multiple myeloma (CARTITUDE-1): A phase 1b/2 open-label study. Lancet. 2021;398:314–324. - PubMed
-
- Munshi NC, Anderson LD, Shah N, et al. Idecabtagene vicleucel in relapsed and refractory multiple myeloma. N Engl J Med. 2021;384:705–716. - PubMed
-
- Gagelmann N, Sureda A, Montoto S, et al. Access to and affordability of CAR T-cell therapy in multiple myeloma: An EBMT position paper. Lancet Haematol. 2022;9:e786–e795. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

