Antioxidant and antiproliferative effect of a glycosaminoglycan extract from Rapana venosa marine snail
- PMID: 38359063
- PMCID: PMC10868805
- DOI: 10.1371/journal.pone.0297803
Antioxidant and antiproliferative effect of a glycosaminoglycan extract from Rapana venosa marine snail
Abstract
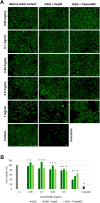
Marine glycosaminoglycans (GAG) isolated from different invertebrates, such as molluscs, starfish or jellyfish, have been described as unique molecules with important pharmacological applications. Scarce information is available on GAG extract from Rapana venosa marine snail. The aim of this study was to isolate a GAG extract from R. venosa marine snail and to investigate its physicochemical, antioxidant and antiproliferative properties for further biomedical use. The morphology, chemical and elemental composition of the extract were established as well as the sulfate content and N- to O-sulfation ratio. Fourier transform infrared (FTIR) spectra indicated that GAG extract presented similar structural characteristics to bovine heparan sulfate and chondroitin sulfate. The pattern of extract migration in agarose gel electrophoresis and specific digestion with chondroitinase ABC and heparinase III indicated the presence of a mixture of chondroitin sulfate-type GAG, as main component, and heparan sulfate-type GAG. Free radical scavenging and ferric ion reducing assays showed that GAG extract had high antioxidant activity, which slightly decreased after enzymatic treatment. In vitro MTT and Live/Dead assays showed that GAG extract had the ability to inhibit cell proliferation in human Hep-2 cell cultures, at cytocompatible concentrations in normal NCTC clone L929 fibroblasts. This capacity decreased after enzymatic digestion, in accordance to the antioxidant activity of the products. Tumoral cell migration was also inhibited by GAG extract and its digestion products. Overall, GAG extract from R. venosa marine snail exhibited antioxidant and antiproliferative activities, suggesting its potential use as novel bioactive compound for biomedical applications.
Copyright: © 2024 Gaspar-Pintiliescu et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
-
- Sharma R, Kataria A, Sharma S, Singh B. Structural characterisation, biological activities and pharmacological potential of glycosaminoglycans and oligosaccharides: a review. Int J Food Sci Technol. 2022; 57(1):4–15.
-
- Abdelmalek BE, Sila A, Krichen F, Karoud W, Martinez-Alvarez O, Ellouz-Chaabouni S, et al. Sulfated polysaccharides from Loligo vulgaris skin: Potential biological activities and partial purification. Int J Biol Macromol. 2015; 72:1143–1151. - PubMed
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

