An antibiotic preorganized for ribosomal binding overcomes antimicrobial resistance
- PMID: 38359125
- PMCID: PMC11665821
- DOI: 10.1126/science.adk8013
An antibiotic preorganized for ribosomal binding overcomes antimicrobial resistance
Abstract
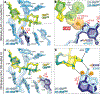
We report the design conception, chemical synthesis, and microbiological evaluation of the bridged macrobicyclic antibiotic cresomycin (CRM), which overcomes evolutionarily diverse forms of antimicrobial resistance that render modern antibiotics ineffective. CRM exhibits in vitro and in vivo efficacy against both Gram-positive and Gram-negative bacteria, including multidrug-resistant strains of Staphylococcus aureus, Escherichia coli, and Pseudomonas aeruginosa. We show that CRM is highly preorganized for ribosomal binding by determining its density functional theory-calculated, solution-state, solid-state, and (wild-type) ribosome-bound structures, which all align identically within the macrobicyclic subunits. Lastly, we report two additional x-ray crystal structures of CRM in complex with bacterial ribosomes separately modified by the ribosomal RNA methylases, chloramphenicol-florfenicol resistance (Cfr) and erythromycin-resistance ribosomal RNA methylase (Erm), revealing concessive adjustments by the target and antibiotic that permit CRM to maintain binding where other antibiotics fail.
Figures




Comment in
-
Ribosome-targeted antibiotic tackles antimicrobial resistance.Nat Rev Drug Discov. 2024 Apr;23(4):249. doi: 10.1038/d41573-024-00040-4. Nat Rev Drug Discov. 2024. PMID: 38448666 No abstract available.
References
-
- Murray CJ, Ikuta KS, Sharara F, Swetschinski L, Robles Aguilar G, Gray A, Han C, Bisignano C, Rao P, Wool E, Johnson SC, Browne AJ, Chipeta MG, Fell F, Hackett S, Haines-Woodhouse G, Kashef Hamadani BH, Kumaran EAP, McManigal B, Agarwal R, Akech S, Albertson S, Amuasi J, Andrews J, Aravkin A, Ashley E, Bailey F, Baker S, Basnyat B, Bekker A, Bender R, Bethou A, Bielicki J, Boonkasidecha S, Bukosia J, Carvalheiro C, Castañeda-Orjuela C, Chansamouth V, Chaurasia S, Chiurchiù S, Chowdhury F, Cook AJ, Cooper B, Cressey TR, Criollo-Mora E, Cunningham M, Darboe S, Day NPJ, De Luca M, Dokova K, Dramowski A, Dunachie SJ, Eckmanns T, Eibach D, Emami A, Feasey N, Fisher-Pearson N, Forrest K, Garrett D, Gastmeier P, Giref AZ, Greer RC, Gupta V, Haller S, Haselbeck A, Hay SI, Holm M, Hopkins S, Iregbu KC, Jacobs J, Jarovsky D, Javanmardi F, Khorana M, Kissoon N, Kobeissi E, Kostyanev T, Krapp F, Krumkamp R, Kumar A, Kyu HH, Lim C, Limmathurotsakul D, Loftus MJ, Lunn M, Ma J, Mturi N, Munera-Huertas T, Musicha P, Mussi-Pinhata MM, Nakamura T, Nanavati R, Nangia S, Newton P, Ngoun C, Novotney A, Nwakanma D, Obiero CW, Olivas-Martinez A, Olliaro P, Ooko E, Ortiz-Brizuela E, Peleg AY, Perrone C, Plakkal N, Ponce-de-Leon A, Raad M, Ramdin T, Riddell A, Roberts T, Robotham JV, Roca A, Rudd KE, Russell N, Schnall J, Scott JAG, Shivamallappa M, Sifuentes-Osornio J, Steenkeste N, Stewardson AJ, Stoeva T, Tasak N, Thaiprakong A, Thwaites G, Turner C, Turner P, van Doorn HR, Velaphi S, Vongpradith A, Vu H, Walsh T, Waner S, Wangrangsimakul T, Wozniak T, Zheng P, Sartorius B, Lopez AD, Stergachis A, Moore C, Dolecek C, Naghavi M, Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. The Lancet 399, 629–655 (2022). - PMC - PubMed
-
- The Review on Antimicrobial Resistance, Antimicrobial Resistance: Tackling a crisis for the health and wealth of nations.
-
- Thomas D, Wessel C, The State of Innovation in Antibacterial Therapeutics. (2022).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

