IC3D Classification of Corneal Dystrophies-Edition 3
- PMID: 38359414
- PMCID: PMC10906208
- DOI: 10.1097/ICO.0000000000003420
IC3D Classification of Corneal Dystrophies-Edition 3
Abstract
Purpose: The International Committee for the Classification of Corneal Dystrophies (IC3D) was created in 2005 to develop a new classification system integrating current information on phenotype, histopathology, and genetic analysis. This update is the third edition of the IC3D nomenclature.
Methods: Peer-reviewed publications from 2014 to 2023 were evaluated. The new information was used to update the anatomic classification and each of the 22 standardized templates including the level of evidence for being a corneal dystrophy [from category 1 (most evidence) to category 4 (least evidence)].
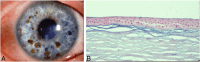
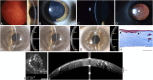
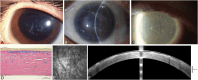
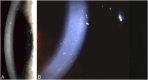
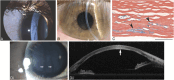
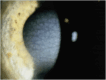
Results: Epithelial recurrent erosion dystrophies now include epithelial recurrent erosion dystrophy, category 1 ( COL17A1 mutations, chromosome 10). Signs and symptoms are similar to Franceschetti corneal dystrophy, dystrophia Smolandiensis, and dystrophia Helsinglandica, category 4. Lisch epithelial corneal dystrophy, previously reported as X-linked, has been discovered to be autosomal dominant ( MCOLN1 mutations, chromosome 19). Classic lattice corneal dystrophy (LCD) results from TGFBI R124C mutation. The LCD variant group has over 80 dystrophies with non-R124C TGFBI mutations, amyloid deposition, and often similar phenotypes to classic LCD. We propose a new nomenclature for specific LCD pathogenic variants by appending the mutation using 1-letter amino acid abbreviations to LCD. Pre-Descemet corneal dystrophies include category 1, autosomal dominant, punctiform and polychromatic pre-Descemet corneal dystrophy (PPPCD) ( PRDX3 mutations, chromosome 10). Typically asymptomatic, it can be distinguished phenotypically from pre-Descemet corneal dystrophy, category 4. We include a corneal dystrophy management table.
Conclusions: The IC3D third edition provides a current summary of corneal dystrophy information. The article is available online at https://corneasociety.org/publications/ic3d .
Copyright © 2024 The Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
The other authors have no funding or conflicts of interest to disclose.
Figures


















Comment in
-
Letter Regarding: IC3D Classification of Corneal Dystrophies-Edition 3.Cornea. 2024 Oct 1;43(10):e28-e29. doi: 10.1097/ICO.0000000000003602. Epub 2024 Jul 11. Cornea. 2024. PMID: 38886891 No abstract available.
-
Reply.Cornea. 2024 Oct 1;43(10):e29. doi: 10.1097/ICO.0000000000003628. Epub 2024 Jul 11. Cornea. 2024. PMID: 38990760 No abstract available.
References
-
- Weiss JS. Schnyder crystalline dystrophy sine crystals. Recommendation for a revision of nomenclature. Ophthalmology. 1996;103:465–473. - PubMed
-
- Groenouw A. Knoetchenfoermige Hornhauttruebungen (noduli corneae). Arch Augenheilkd. 1890;21:281–289.
-
- Biber H. Ueber einige seltene Hornhauterkrankungen: die oberflaechliche gittrige Keratitis [Inaugural Dissertation]. A Diggelmann Zuerich; 1890.
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Miscellaneous

